Table of Contents
Selective flocculation utilizes the differences in the physical-chemical properties of the various mineral components in the mixed suspension. It is based on the preferential adsorption of an organic flocculant on the particular solids to be flocculated, leaving the remainder of the particles in suspension. In order to understand the mechanics of this process, selective flocculation may be divided into four major sub processes; these being: ore slurry dispersion, in which all the particles are stably and uniformly distributed in the suspension with the individual particles being essentially separate; flocculant selective adsorption and floc formation; floc conditioning, which aims at obtaining flocs with desire properties for their subsequent separation and with minimum entrapment of dispersed particles; and floc separation from the suspension.
Design of Selective Flocculation Processes
In designing a selective flocculation process to separate certain desired particles from mixed suspensions with unwanted (gangue) solids, selective flocculation of either the valuable components or the gangue components may be employed depending on which route is more technically and economically viable. Both of these routes have been successfully applied on a commercial scale. More specifically to selective flocculation, the choice of either route would be influenced by the available knowledge of the surface and flocculation behaviors of each of the components under various environments of reagents suite. Generally, the route that would result in a lower reagent consumption, more efficient separation and lower equipment cost (both capital and operating cost) would obviously be the preferred choice.
As mentioned earlier, there are four sub processes in selective flocculation which make up the total process. These are, dispersion of ore suspensions; selective adsorption of flocculant and floe formation; floc conditioning; and floc separation from suspension.
Most of the attention of published research has been understandably directed towards the selective adsorption of flocculant and floe formation, since there cannot be a separation without the selective adsorption of flocculant on the desired particles of the ore suspension.
Although the importance of the chemical aspects of flocculant adsorption and floc formation cannot be over emphasized, the effects of hydrodynamic conditions in the agitation vessel and other process parameters are also important. Since the flocculant is likely to adsorb on more than one particle simultaneously, floc formation might be considered an almost inseparable step from adsorption. Subsequent floc growth and breakup, however, is largely governed by the hydrodynamic conditions and particle size distribution.
Applications of Selective Flocculation
A number of successful applications for selective flocculation have been demonstrated in the laboratory and pilot plant but with only a few commercial applications. At present, there are only two known commercial applications for selective flocculation in North America.
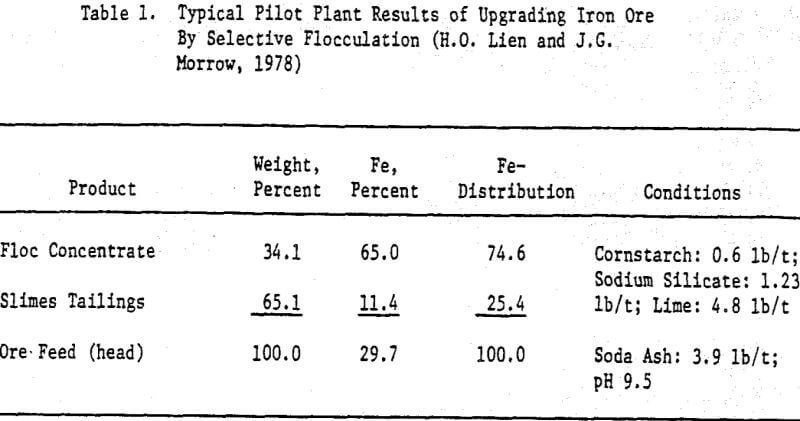
Selective flocculation was induced by the preferential absorption of a small amount of causticized starch on the iron oxides. Sodium hydroxide was added to bring the pH to 10.5 to 11, and sodium silicate was used as a dispersant. The ore had to be ground to below 400 mesh to release the iron minerals. The concentrate contained 62 to 64 percent Fe, and 4.5 to 5.5 percent SiO2. Iron recoveries were 75 to 80 percent. Causticized tapioca starch and dextrin were used for suppressing the iron minerals in the flotation step, where a cationic collector Arosurf MG-98A and Dow Froth 250 were used for flotation of gangue minerals. Lime, CaCl2 and flocculant SF330 were used for treating the recycled water.
Use of selective flocculation to intensify magnetic separation in strong and weak fields. By preparing the pulp through dispersion and selective flocculation before magnetic separation, extraction of iron into the concentrate can be greatly increased without any loss of quality of the latter. The reagents recommended were caustic soda (1.0 kg/ ton), water glass (sodium silicate) or CSL waste (0.4 or 0.7 kg/ton), and synthetic polymers (7 to 10 g/ton).
Evidence for selectivity of flocculation with synthetic mixtures of finely-divided minerals has been obtained. For example, xanthate containing polymers (namely cellulose, cellulose derivatives and PVA xanthates) showed marked selectivity towards minerals like galena, pyrite, chalcopyrite and chrysocolla while they had little or no flocculating effects on calcite, quartz, feldspar and kaolinite. These polyxanthates almost fulfill the ideal case of yes- or-no adsorption. With these specifically adsorbing polymers, selective flocculation of chrysocolla from quartz and of galena from calcite could be achieved. However, it was found that soluble Cu²+ ions can activate mixtures of feldspar and kaolite, therefore rendering the polyxanthate flocculant unselective.
Sodium hexametaphosphate dispersed quartz with greater stability than it did chrysocolla. Only the non-ionic flocculant, in the presence of the dispersant, was useful for flocculating chrysocolla. None of the flocculants had an effect on the quartz. The addition of a small amount of NaCl improved the selectivity of separation. High solids content reduced the efficiency of separation due to entrapment of gangue in the flocs. Malachite-quartz mixtures did not prove to be amenable to separation by selective flocculation.
The U.S. Bureau of Mines investigated a reagent scheme similar to Cominco’s using Carlsbad sylvinite ores containing 5 percent insoluble slimes. The insoluble slimes were different than those present in the Cominco plant feed because they contained appreciable quantities of montmorillonite clay as well as magnesite, anhydrite, calcite, and iron oxides. Laboratory test results showed that at least 85 percent of the insoluble slimes had to be removed to obtain an 85 percent potash recovery; below 80 percent slimes removal a drastic decrease in potash recovery was observed.
Selective flocculation of kaolin clay from rutile and hematite was achieved. The process consisted of deflocculating an aqueous suspension of the impure clay by adding an alkali metal silicate. The clay particles were then selectively flocculated by adding a synthetic organic poly-anionic flocculating agent, such as weakly anionic partially hydrolyzed polyacrylmide. A concentrate of deflocculated impurities was then separated. The pH of the suspension was adjusted to 9.5 to 11.5 by adding an alkali metal hydroxide before adding the silicate. Then before adding the flocculating agent, the pH was reduced to 7.0 to 8.8 by addition of acid. An improvement of clay purity and brightness was realized..
In order for these applications to be successful, understanding of the process parameters in a more quantitative fashion as well as the development of new reagents and appropriate equipment should be made. Research along these lines is currently being made by this author and his coworkers, using different ore types to investigate the feasibility of this technology.

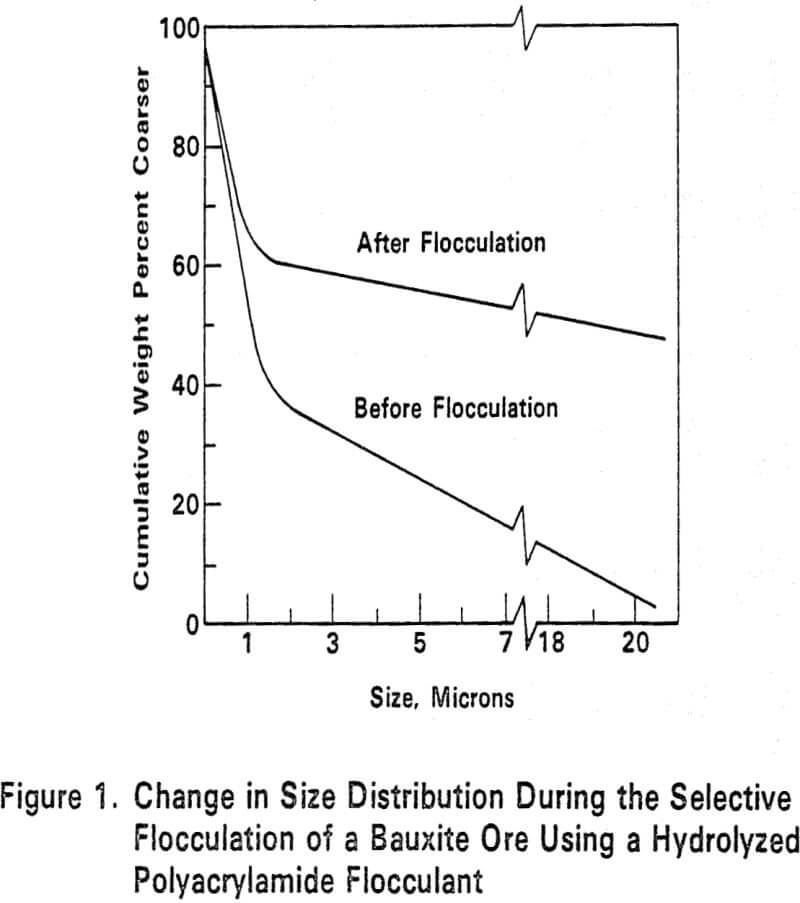

Selective Flocculation Process Design
Selective Flocculation process development generally involves single mineral flocculation tests to identify the appropriate polymer and experimental conditions for achieving the desired selectivity. Invariably the selectivity predicted on the basis of such tests is not realized when mixed mineral tests are conducted. A systematic experimental investigation revealed that adsorption of the polymer on various substrates is critical to selecting the suitable flocculent. Simultaneously, a mathematical model was developed to predict the overall process efficiency with a given polymer. In this paper, results of these efforts to scientifically design a suitable selective flocculation process are presented.
Dispersion of the Mineral Suspension
In selective flocculation process obtaining a stable dispersion of the mixture is the first step. Dispersion may be achieved either by mechanical or surface chemical forces. Since the surface chemical forces are much stronger in nature as compared to the mechanical forces, they are more widely used. A pre-requisite to dispersion is complete liberation of the value from the gangue.
The zeta potential of the single mineral component of the ore is commonly determined to select dispersion conditions by manipulating the surface charges. Typically the pH selected must be such that the zeta potential of the particles is of the same sign for all the components and the magnitude is greater than 25 mV, since suspension with lower zeta potential values are unstable. Also in some cases dispersants like sodium silicate, sodium hexa-meta phosphate are used. Silicate ions (and other similar ions) act as specifically adsorbing ions which can change the surface characteristic/charge of one or more component of the ore. For example apatite can be dispersed at pH 9.5, only in the presence of sodium silicate.
Adsorption of the Flocculent and Floc Formation
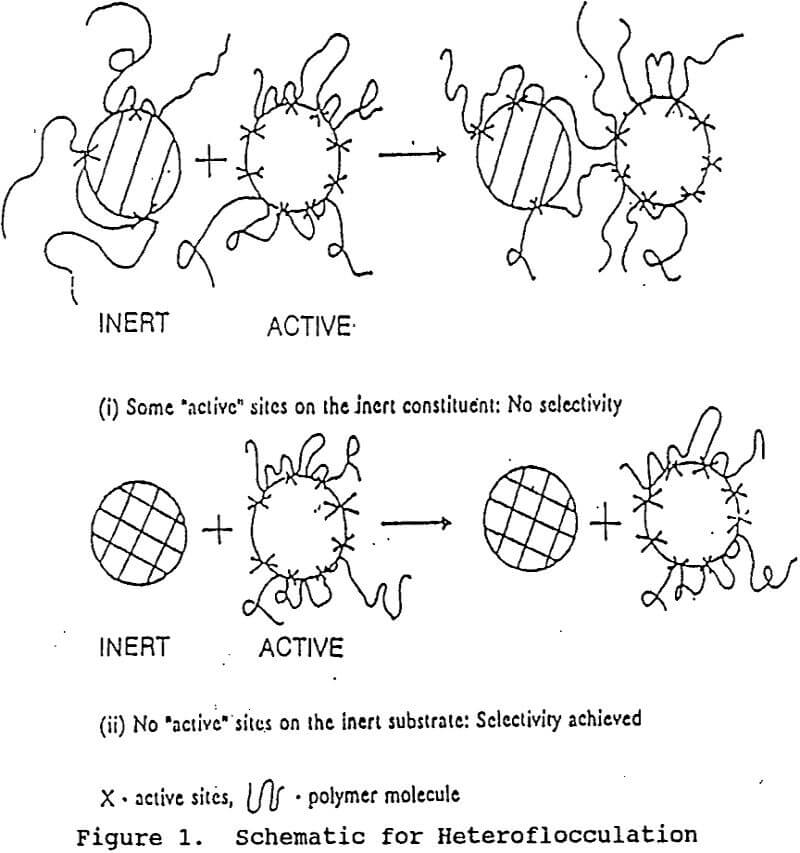
The major concern in this step is to achieve selective adsorption of the polymer on the active component (value or gangue material). Typically a flocculent capable of causing bridging flocculation is selected. These may be ionic or non-ionic in nature and are straight chain molecules of high molecular weight (greater than 1 million). A proper selection of the flocculant requires understanding of the polymer adsorption onto particulate/water interface.
The polymer adsorption mechanisms may be governed by either the chemical forces or physical forces or a combination of the two. The physical forces such as electrostatic attraction, London van der waals attraction, hydrophobic attraction or bonding and hydrogen bonding (with low heats of adsorption), are mostly non selective. On the other hand, the chemical forces are very specific. These may be covalent bonds, complex co-ordinate bonds or hydrogen bonds characterized by a very high heat of adsorption.
Separation of the Flocs from the Dispersed Slurry
Separation of the flocs from the dispersed slurry would depend on the characteristics of the flocs such as size, density (or fractal dimension). In general the separation is achieved by either gravity settling of by floc flotation. The choice of the separation method would also depend on the density of the solid in the dispersed slurry. For example in coal (sp. gr. 1.3) and coal pyrite(sp. gr. 4.9) separation, if coal is flocculated then the flocs are separated by flotation in spite formation of large flocs of coal.
It should be recognized that in separation of the flocs by gravitational settling an appreciable amount of the dispersed slurry also settles due to entrainment. However separation efficiency can be improve by re-suspending the flocs after agitating at a lower/same speed to minimize the floe breakage. In case of physical entrapment of the inert particles, the flocs need to be broken and then re-flocculated to improve the process efficiency.