Waste products from the chlorination of rutile present a serious disposal problem because of the toxicity of some of the metallic ions when waste residues are dissolved in water and HCl gas is released into the atmosphere. Previous work showed that the water-soluble portion of the chlorination waste contained chloride and chloride-oxide complexes of Cb, Ta, Ti, Zr, Al, Cr, Fe, V, and minor amounts of other elements. The insoluble residue was rutile and coke, which could be recycled to a chlorinator to produce TiCl4.
Estimates based on information supplied by several companies indicated a solid-waste generation of 50,000 tons per year. This waste contains 1,000 tons of columbium and 200 tons of tantalum. This report describes a method of separating a columbium-tantalum fraction from the titanium-zirconium fraction in the water-soluble portion. Leaching tests, using chlorinator solid-waste products produced at industrial plants of the companies mentioned in footnote 4, showed that samples from three of the companies were very similar, and that samples from the fourth company were largely FeCl3.
Experimental Procedures and Results
Initial attempts to separate elements from the leach liquors by solvent extraction were not successful because of the large amount of FeCl3 present in the leach liquors which extracted before the other ions could be extracted. Pressure hydrolysis of the leach liquors selectively precipitated Cb, Ta, Ti, and Zr. This precipitate was soluble in dilute H2SO4 and in HCl-HF mixtures. Attempts to selectively precipitate the zirconium as a phosphate from the acid solution were not successful, so solvent extraction procedures were used to separate the columbium and tantalum from the titanium and zirconium
Preliminary tests using methyl isobutyl ketone indicated that there were significant losses of the solvent when in contact with the aqueous phase and upon stripping the organic phase. Published data give the solubility of methyl isobutyl ketone as 2 pct in water, and the solubility of Amberlite LA-1 as 10 to 20 ppm in water. Hunter had miscibility problems with organic and aqueous phases in his use of other ketones. Bielecki used solid ion exchange for separating columbium and tantalum. Systems composed of secondary amines in hydrocarbon carriers we’re used for this study.
A composite leach liquor was prepared by water leaching solid wastes from three of the companies mentioned in footnote 4. Analysis of a typical composite leach liquor, in g/l, was as follows: Ti, 5.6; V, 15.2, Cr, 5.46; Fe, 15.3; Cb, 7.7; Zr, 22.3; Al, 7.8; and Ta, 2.0. Boiling the leach liquor produced hydrolysis and partial precipitation of the Cb, Ta, Ti, and Zr. However, the separation was not complete even after the boiling and filtering sequence was repeated five times. The heat and pressure that resulted from hydrolysis at 130° to 200° C produced a virtually complete precipitation of Cb, Ta, Ti, and Zr. Table 1 shows the effect of a given temperature on the separation at a time of ½ hr. Increasing the temperature from 130° to 180° C decreased the precipitation of tantalum by 3 pct. Therefore, 130° to 150° C was used for all other hydrolysis tests.
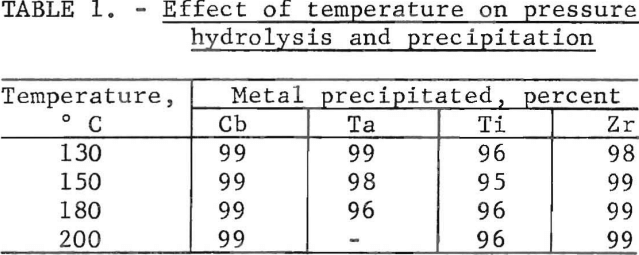
Filtration of the solids that contained the Cb, Ta, Ti, and Zr from the liquid portion was accelerated by adding a small amount of H2SO4 to the leach liquor before hydrolysis. One part H2SO4 to 120 parts leach liquor reduced the filtration time from 7 hr to 7 min. As shown in table 2, changing the ratio of H2SO4-to-leach liquor from 1-to-120 to 1-to-30 caused a decrease in the precipitation of both columbium and tantalum when operated at 150° C. Decreasing the ratio below 1 to 120 prolonged the filtering time; for example, 1 part H2SO4 to 250 parts leach liquor extended the filtering time to 4 hr.
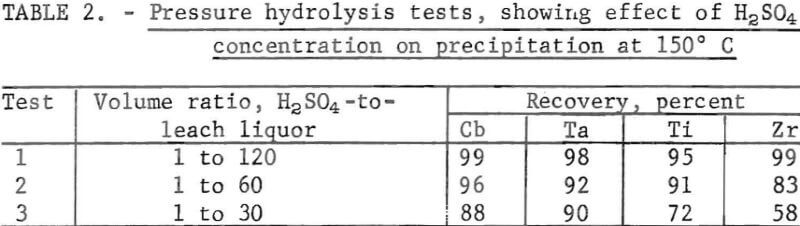
Several 1,000-g batches of hydrolyzed solids of Cb, Ta, Ti, and Zr were dissolved in 4.5 N H2SO4 and in HCl-HF (table 3). Secondary amine was used for extracting columbium and tantalum from titanium and zirconium. The columbium and tantalum extractions were highest from the HCl-HF mixture, as shown in table 3, therefore the HCl-HF mixture was used in further studies. Organic-to-aqueous ratios were 1 to 1 in all tests described by this report.
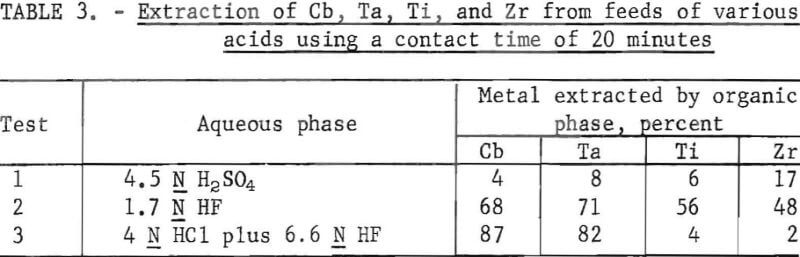
Data in table 4 compare the extraction by the secondary amine from feeds of various HCl-HF combinations. The best extraction of columbium and tantalum occurred in the feed with the highest acid concentrations (test 1) although the rejection of titanium and zirconium was superior in other acid combinations. A contact time study was made using a 4 N HCl and 6.6 N HF feed. The results are shown in table 5. The best extraction of columbium and tantalum occurred in 1 min.
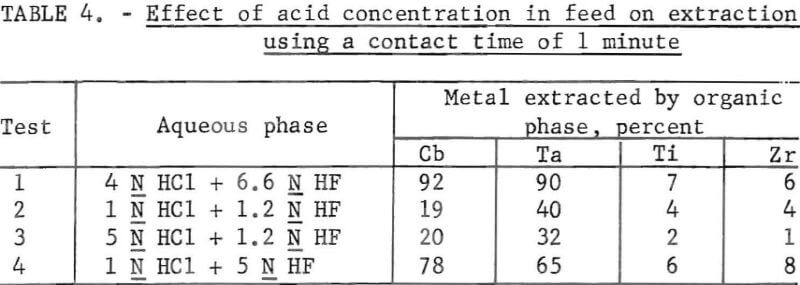
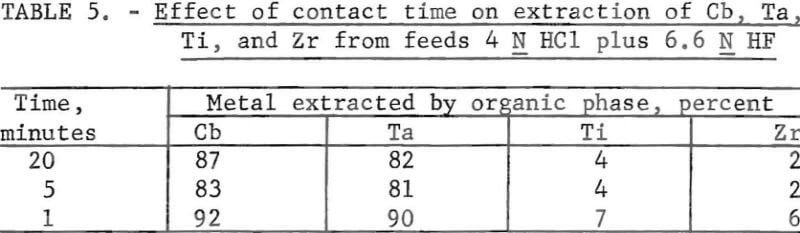
Various stripping agents for the removal of the extracted elements from the loaded organic phase were tested. Portions of a previously prepared loaded solvent that contained columbium and tantalum were used for the stripping tests. An aliquot of the organic phase was contacted four times for 20 min with fresh volumes of the stripping solution. NH4OH was added to each separated strip solution to obtain a pH of 8 to 9. The columbium and tantalum were precipitated from this solution and filtered, and the precipitate was ignited and weighed. Results are shown in figure 1. Water was the least effective stripping agent; 1 N HCl and 1 N NH4Cl were equally effective. The addition of 20 ml of concentrated HF to 1 liter of 1 N HCl decreased stripping efficiency.
Initial studies showed that a small amount of third-phase emulsion formed while using the secondary amine in a kerosene carrier for separating columbium and tantalum from titanium and zirconium. In preparing a large quantity of the loaded organic phase for stripping studies, the volume of the third-phase emulsion amounted to 19 vol pct of the organic phase. Two vol pct of isodecyl alcohol in the amine kerosene decreased third-phase formation, but had to be replenished about every third recycle of the extractant.
The organic extractant must be reusable in order for the process to be practical. To prove this, a 500-ml batch of kerosene-amine-isodecyl alcohol was used to extract a feed solution. This organic phase was then stripped four times with 1 N HCl, and the organic phase was used to extract more feed solution. This was repeated a third time. The columbium and tantalum extractions were approximately the same as those reported in table 4, test 1.
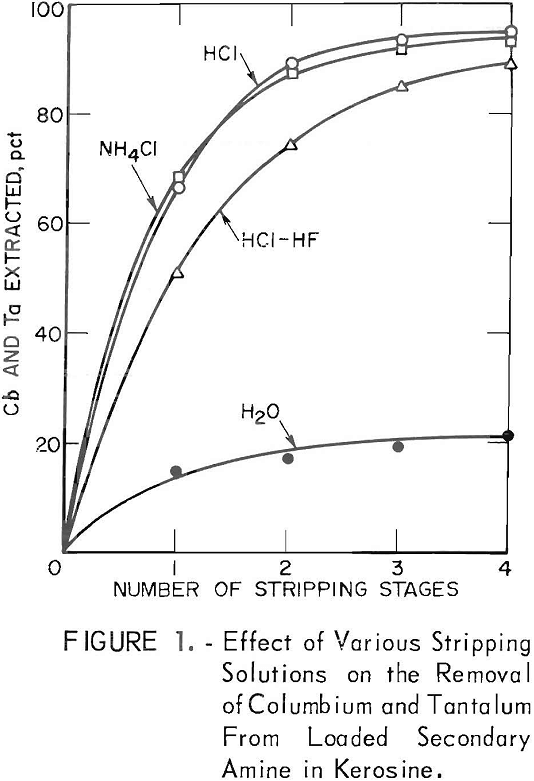
A final scrub of the organic phase indicated that there was no buildup of any of the elements. Continued recycling of the organic phase in a kerosene carrier required periodic replacement of the isodecyl alcohol to prevent third-phase formation. The emulsion phase did not form with either xylene or Socal 355-L. Carriers that contained the secondary amine were contacted once for 20 min with the aqueous feed and then were stripped four times with separate volumes of 1 N HCl solution. Total weight-percent extracted is shown in figure 2. The columbium and tantalum were removed from the xylene carrier more completely than from the other carriers.
Continuous Countercurrent Extraction
The method that was developed for separating columbium and tantalum from titanium and zirconium was tested in a 10-stage extractor. The miniature pump mixer-settler extractor used in this investigation was developed by Bauer, Lindstrom, and Higbie; Borrowman and Rosenbaum developed a similar unit. The 10-stage extractor is shown in figure 3. Each stage was fabricated from a piece of Lucite 5-½ by 3-½ by 1-5/8 in thick. The settler section holds about 80 cm³ below the weir, and the mixer section holds about 15 cm³. The propellers and shafts are Teflon-coated stainless steel and are motor-driven by an endless belt and pulleys. Each stage is a mirror image of the adjacent stage, separated by 1/15 in of hard Teflon. The Teflon separators are slotted, allowing the solution level to be controlled by a gate that covers the slot. Flow rates were controlled by tubing pumps at about 20 ml/min with a phase ratio of 1 organic to 1 aqueous. The feed was an autoclave precipitate dissolved in 4 N HCl and 6.6 N HF. The feed contained approximately 4 to 8 g/l Cb plus the other ions. The extractant was 0.25 molar Amberlite LA-1 in an organic carrier.
Tests were made with a kerosene carrier that contained 2 vol pct isodecyl alcohol as a modifier. A third phase was encountered and was partly corrected by reducing the feed rates to allow more residence time to promote better phase separation. The recoveries were lower than expected when compared with earlier tests using separatory funnels. Increasing the isodecyl alcohol
content of the carrier to 8 vol pct decreased the third-phase formation, but the recoveries were not improved. The use of modifiers often decreases extraction coefficients. Analysis of raffinates and strips are shown in table 6. Data show that approximately 90 pet of the columbium and 80 pct of the tantalus were extracted by the kerosene and secondary amine mixture along with 1 to 3 pct of the titanium and zirconium. Phase separation that used kerosene was a problem in all tests that used the 10-stage reactor. Xylene did not form a third phase, and data in figure 2 showed that xylene was more completely stripped, so it was used in subsequent tests as a carrier. The progress of 3 1 of feed through the countercurrent 10-stage reactor was studied by taking samples that represented overflow increments of 500 ml. This represented an approximate feed rate of 20 ml/min. The operational stability of the extractor was satisfactory, and the average analysis of five 500-ml portions was relatively constant as shown in table 6.
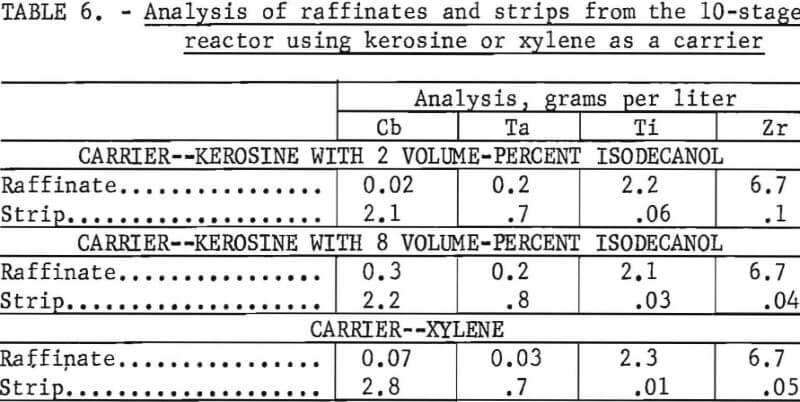
Three liters of 1 N HCl were pumped through the 10-stage reactor countercurrent to the loaded organic phase in the same manner as the feed. The reactor was filled in 25 min, and the feed rate was 24 ml/min. The progress of the stripping solution, sampled in a manner similar to that used with the feed, indicated a consistent stripping rate. The analyses for the raffinates and strips are shown in table 6. The results show that more than 95 pct of the tantalum and columbium was extracted by the secondary amine in a xylene carrier and that less than 1 pct of the titanium and zirconium was extracted.
Conclusions
Tests have shown that Cb, Ta, Zr, and Ti can be separated from waste residues produced by the chlorination of rutile. Waste products were leached with water and filtered, giving a filtrate containing Cb, Ta, Zr, Ti, Cr, V, Al, and Fe. Pressure hydrolysis separated the Cb, Ta, Zr, and Ti from the other elements as a white precipitate. This precipitate is soluble in aqueous HCl-HF. Solvent extraction using a secondary amine was shown to be a satisfactory method of separation.
