Table of Contents
As part of our mission to investigate technology options for the development of domestic mineral resources, conducted tests aimed at separating the copper-nickel values in bulk sulfide mattes into copper-rich and nickel-rich fractions. Resources in the Duluth Gabbro Complex are estimated to be 4.4 billion short tons of mineralized rock grading more than 0.50 pct Cu and 0.15 pct Ni. In addition to copper and nickel, this deposit is also a significant resource of cobalt, platinum-group metals, silver, and gold. Major metallurgical problems associated with these ores are separating the copper from nickel, recovering cobalt, and determining the distribution of platinum-group and other precious metals. This report describes the results obtained from separating mattes smelted from Duluth Gabbro bulk flotation concentrates into copper-rich and nickel-rich fractions by mineral dressing techniques.
In conventional processing of mixed sulfide ores of copper and nickel, the separation of copper from nickel begins in the ore beneficiation plant. Although copper and nickel are mutually soluble in the metallic state, in sulfide ores they occur as distinct minerals, usually chalcopyrite (CuFeS2) and pentlandite (FeNi)8S4). The separation of copper and nickel is achieved by differential flotation, if the mineral grains can be liberated by grinding. Normal practice is to depress the nickel sulfides by increasing the pH of the flotation pulp to between 9 and 11 and selectively floating the copper minerals. The Duluth Gabbro Complex ores are unique in that they contain higher copper-to-nickel ratios (3:1 to 4:1) than do the Sudbury, Ontario, ores (<1:1). In addition, up to 50 pct of the copper in the Duluth ores occurs as cubanite (CuFe2S3). The flotation characteristics of cubanite are quite similar to those of pentlandite, so the production of nickel fractions low in copper content is difficult.
At the INCO (International Nickel Co.) operation in Sudbury, Ontario, further separation of nickel from copper is accomplished by application of mineral dressing techniques to slow-cooled nickel mattes. These mattes contain about 50 pct Ni, 30 pct Cu, 20 pct S, and <1 pct Fe. They consist of grains of chalcocite (Cu2S) in a matrix of heazlewoodite (Ni3S2), plus small amounts of copper-nickel metallics. The mattes are slow cooled for 4 days, increasing the grain size of the mattes, which improves the sharpness of the subsequent separation. After the mattes are ground to liberate the sulfides, the copper-nickel metallics, which contain a high concentration of precious metals, are removed by magnetic separation. Then the copper sulfides are floated from the nickel sulfides to produce separate copper and nickel fractions., The object of the matte separation at INCO is to produce high-grade nickel concentrates at the expense of producing somewhat contaminated copper concentrates. The nickel concentrates are sufficiently low in copper to allow the reduction of the nickel sulfides to metallic anodes for subsequent electrorefining. The copper concentrates, on the other hand, are relatively high in nickel (5 to 6 pct) and are recycled to the copper flash smelter for processing to blister copper.
In contrast to the Sudbury mattes, the mattes smelted from Duluth Gabbro Complex bulk concentrates are much higher in copper (60 pct) and contain only about 8 to 10 pct Ni. To improve cobalt recovery, about 5 pct Fe was left in the mattes, which strongly affected their mineralogical composition when solidified. Rather than having individual grains of chalcocite in a matrix of heazlewoodite, the mattes consist of a primary structure of intergrown bornite (Cu5FeS4) and chalcocite grains with stringers of heazlewoodite between the primary grains. The mattes also contain small amounts of an iron-nickel alloy rather than the copper-nickel metallics present in the INCO mattes.
Separation of these mattes into fractions similar to those produced at Sudbury would not be feasible because of the major differences between the matte structures as well as the relative proportions of copper and nickel present in them. Therefore, the objective was to separate a copper-rich fraction sufficiently low in nickel to allow processing of the copper to anodes for electrorefining and a nickel-rich fraction with a nickel-to-copper ratio of at least 1, for processing in a hydrometallurgical circuit to recover the nickel, cobalt, precious metals, and remaining copper.
Raw Materials
Matte Preparation
The mattes for this study were prepared from lots of bulk copper-nickel sulfide concentrates. These concentrates were described in detail in a previous report. Processing consisted of pelletizing, roasting, and smelting the concentrates to recover a matte containing about 60 pct Cu, 8 to 10 pct Ni, 5 pct Fe, and 20 pct S. Analyses of a typical matte were as follows:
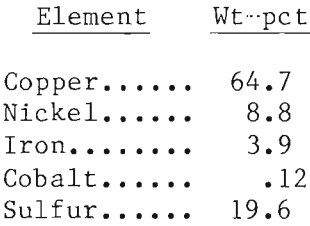
The mineralogical composition of these mattes was investigated in some detail prior to their actual separation (table 1). The matte consisted of a matrix of intergrown chalcocite and bornite with stringers of heazlewoodite between the grains of the matrix. Frequently, the heazlewoodite was associated with metallic grains, which proved to be either an iron-nickel alloy or metallic copper (fig. 1).
The grain size of the solidified mattes appeared to be in the range of 100 to 500 µm for the copper sulfides, while the nickel sulfides that filled the voids between the chalcocite-bornite grains ranged from 50 µm or less in width up to about 100 µm. However, because of the structure of these mattes, physical liberation of the nickel from the copper sulfides did not appear to be closely related to grain size.
An electron probe microanalysis of the individual grains was also carried out to determine the composition of the sulfides and metallics (table 1). At this matte composition, iron was found to associate with the nickel only in the metallic state and with copper only in the sulfide state. The iron-nickel alloy proved to be ferromagnetic and could be recovered quite readily by magnetic methods.

Slow Cooling
Because increased grain size in the matte was expected to enhance the separation efficiency, several lots of mattes were slow cooled for periods ranging from 6 hours up to 2-½ days. These cooling cycles were accomplished by remelting 1,000-gram lots of matte at 1,200° C in clay-graphite crucibles with graphite covers, in a programmable muffle furnace, and cooling the crucibles in the furnace from 1,200° to 350° C over a set time period. A nitrogen atmosphere was maintained in the furnace throughout the melting and cooling cycles. Three crucibles, each containing 1,000 grams of matte, were slow cooled during each furnace cycle. The contents of the crucibles were crushed, combined, and blended, producing sufficient matte for 10 laboratory flotation tests.
Laboratory Procedures
The experimental flotation procedure evolved through several stages before a final simplified and reproducible version was developed. The version selected, after extraneous magnetic separations and middlings recirculations were eliminated,
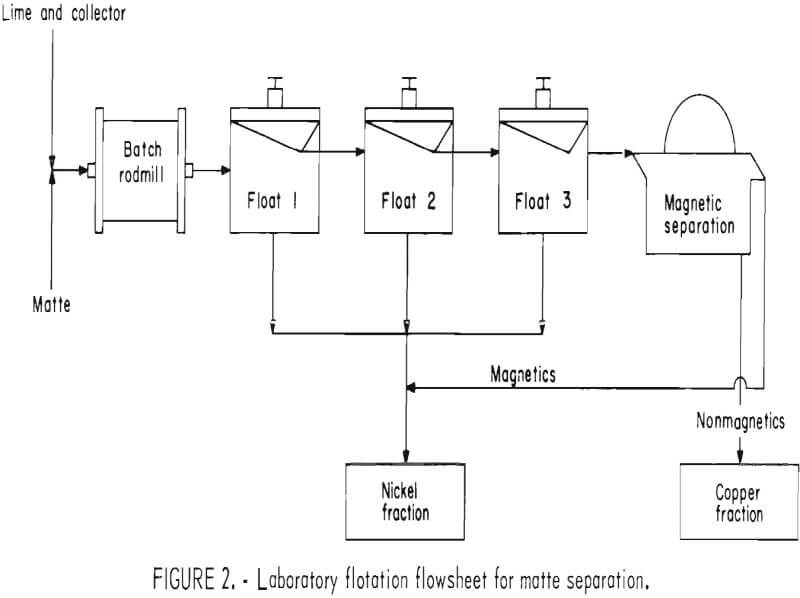
is represented by the flowsheet in figure 2.
Batch grinding was followed by one rougher (float 1) and two cleaner flotation steps (floats 2 and 3), with the final froth being cleaned magnetically to remove any iron-nickel alloy that carried through the flotation. Collectors that were insoluble in water were added to the grinding mill, as shown in figure 2; those that were water soluble were added to the laboratory flotation cell. Frother, when required, was added to the cell just before flotation. Each separation product was filtered, dried, weighed, and analyzed individually. The float 1, float 2, and float 3 tailings and the magnetics were then combined to constitute the nickel fractions.
Results
Flotation Collector
Several flotation collectors were evaluated throughout the course of the experimental work. Those selected
included diphenylguanidine (DPG), potassium ethyl xanthate (KEX), isopropyl ethylthionocarbamate (IEC), Minerec 1661, and sodium isopropyl xanthate (NIX). The collectors DPG, IEC, and Minerec 1661 exhibited sufficient copper selectivity to yield the sharpness of separation required to produce satisfactory copper and nickel fractions (table 2). No nickel depressant other than lime was used throughout the test work. Saturated lime solutions demonstrate a pH level of 12.4 to 12.5. Since nickel sulfide depression is highest at this pH level, most testing was carried out in saturated lime water.
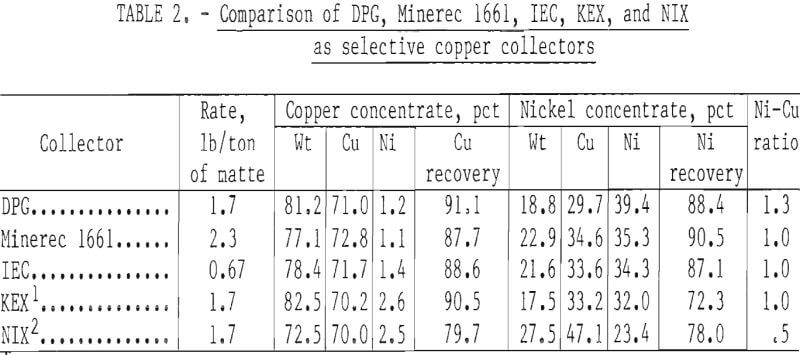
At pH 12.4, KEX did not float the copper sulfides. When flotation was carried out at lower pH’s (8 to 10), the separation of copper from nickel was not sharp. Sodium isopropyl xanthate (NIX) was also not an effective copper collector at pH 12.4 and lacked selectivity at lower pH levels.
Minerec 1661 and IEC demonstrated good selectivity at pH 12.4 and produced adequate copper and nickel fractions, although the nickel-to-copper ratio in the nickel fractions was not above 1.0. Diphenylquanidine (DPG) was as selective as IEC and Minerec 1661 and gave higher copper recoveries in the copper fractions. Minerec 1661, DPG, and IEC are all relatively insoluble in water and were diluted to 5-pct solutions with ethanol for convenient additions. Adding these collectors to the flotation cell was not effective, in spite of conditioning times of up to 30 minutes. Effective flotation responses from these reagents required adding them to the grinding mill. (The water-soluble xanthates were not tested in the grinding mill because they were not sufficiently copper- selective in the preliminary tests.) Since DPG has moderate frothing properties, no additional frother was required when this collector was used. A lack of froth in the second and third floats was remedied by recycling the filtrate water from each previous flotation step. This returned unadsorbed DPG to the cell and effectively increased the froth volume.
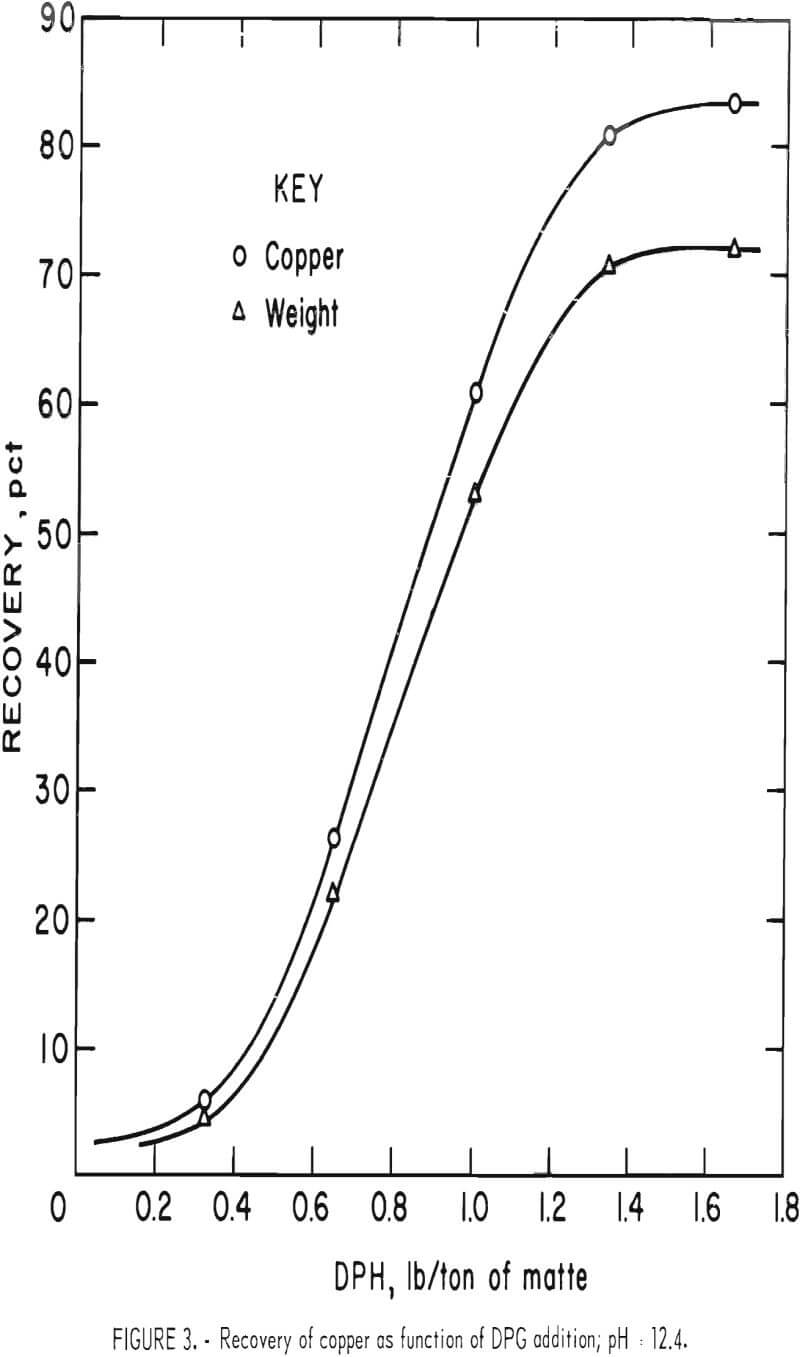
Since DPG produced the best results of any of the collectors tested, most of the flotation was carried out with it, and an effort was made to find the best addition level. Figure 3 shows the cop-per recovery with various levels of DPG addition. The results are for the rougher (first) float only, with 30-minute grinding periods. The sharpness of the flotation endpoint is demonstrated by the smoothness of the recovery curve. This was the only collector that demonstrated a sharp, clear endpoint. It can be seen that the recovery curve flattens at 1.7 pounds DPG per ton of matte and increases only slightly above this level.
Effect of Slow Cooling on Matte Structure
Samples of matte from the slow-cooled series were examined petrographically to determine the effects of slow cooling on the matte structure. As mentioned previously in the section “Matte Preparation,” the mattes as cast consisted of a primary structure of intergrown chalcocite-bornite grains with stringers of heazlewoodite between the primary grains. The copper sulfides are the first phases to solidify, beginning at about 900° C (depending on the iron content of the melt), followed by the iron-nickel alloy and copper. The final phase to appear is the nickel sulfide, which solidifies at 575° C. To insure completion of the slow cooling process, the cooling cycle was continued to 350° C.
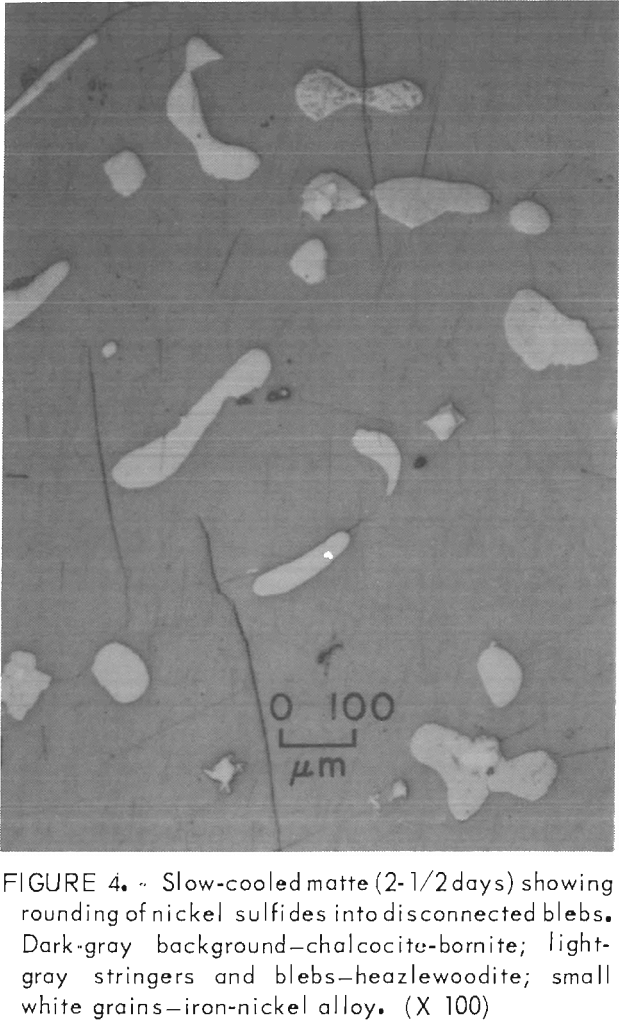
Upon prolonged cooling (2-½ days), the primary grain structure increased slightly in size, and the heazlewoodite stringers tended to coalesce into rounded blebs (fig. 4). The metallic grains were relatively unaffected by the slow cooling process, except that the quantity of metallic particles may have increased slightly. This could be attributed to a decrease of about 0.5 pct in the matte sulfur content during the remelt and the slow cooling process. Overall, the slow cooling process did not have the dramatic effect on the grain size of mattes of this composition that it does on mattes in which the volume concentration of heazlewoodite exceeds that of chalcocite.
Effect of Slow Cooling on Flotation
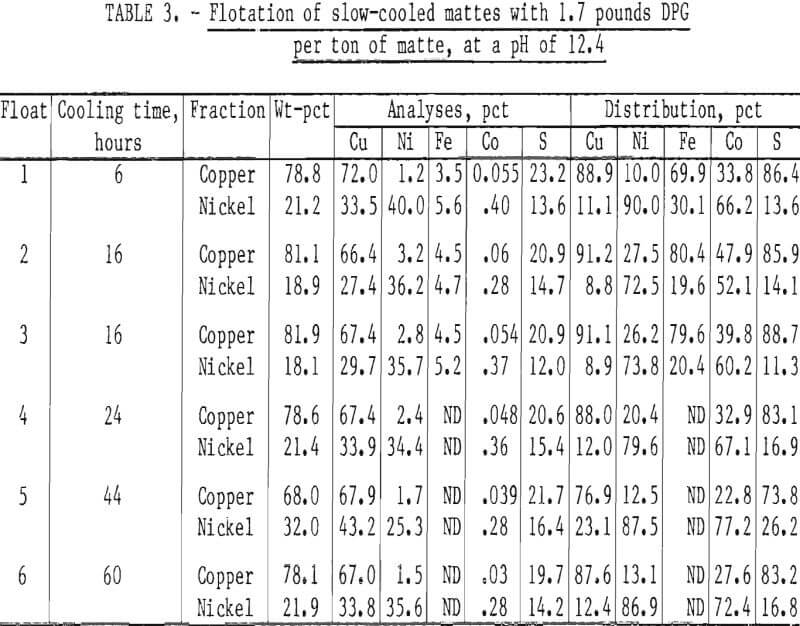
Several series of tests were run on each of the slow-cooled samples (table 3). The standard procedure previously described and shown in figure 2 was applied uniformly to each sample. The table shows that the results for the 2-½ day slow-cooled mattes were not better and were possibly a little poorer than the results obtained with the fast-cooled mattes. This result was unexpected, because the literature points out the advantages of slow cooling. The best explanation is that when the fast-cooled mattes were ground, the mattes broke between the chalcocite-bornite grains, and continued grinding scrubbed the heazlewoodite from the surface of the copper sulfide grains. With slow cooling, the heazlewoodite coalesced into rounded, discontinuous blebs. Upon grinding, fractures occurred through the primary chalcocite-bornite matrix, but the heazlewoodite remained trapped in the matrix. In any case, cooling times beyond what would be normal for casting these mattes did not appear to be beneficial or necessary to meet our objectives.
Flotation of Mattes with DPG
After the testing of various collectors, most of the work was performed using DPG as both collector and frother, and lime for pH control. No nickel depressants other than lime were used. The procedure was the same as that developed for collector evaluation, namely, one rougher (float 1) and two cleaner flotation steps (floats 2 and 3) followed by a magnetic cleaning of the second froth to remove any iron-nickel metallics that had carried through the flotation steps. Each flotation test was carried out with four successive batches of 300 grams of matte. The individual copper and nickel fractions from each batch were dried and weighed. If the weights of the respective fractions were relatively consistent from batch to batch, the respective fractions were combined and the composited fractions treated as a single test product. This helped to average the variations from batch to batch and reduced the number of chemical analyses required for each test. Inconsistent results within a four-batch test necessitated repeating the test.
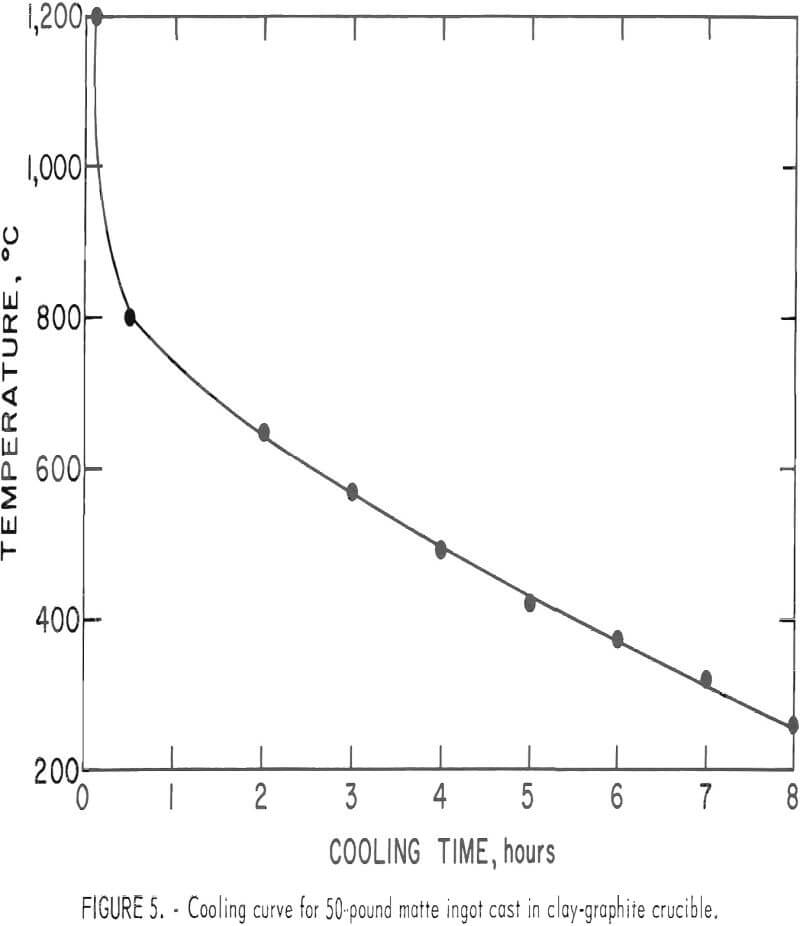
One of the main concerns of this work was to monitor the consistency of the results from one lot of matte to another. To determine if differences caused by experimental variations during smelting and solidification would have a significant effect on flotation, mattes from two lots were floated according to the procedure shown in figure 2. Both lots of matte were processed without any slow cooling procedure other than being cast into sand-supported fire-clay crucibles. The 50-pound ingots of matte were completely solidified within 30 to 40 minutes and were left to cool in the crucible (fig. 5). Structures of these mattes were similar to those that were cooled from 1,200° to 350° C in 6 hours.
Comparison of the results (table 4) shows that the separation of copper and nickel was equivalent for both mattes, even though matte I contained less sulfur.
Effect of pH on Flotation Response
To determine if matte separation could be accomplished at lower pH’s than that of saturated lime solutions (12.4), a series of tests was carried out with pH’s ranging from that of the natural flotation pulp (7.7) to 12.3 (table 5). The most significant finding in this test series using DPG as the collector was that the recovery of copper improved as the pH increased. This trend is opposite to the results obtained with sulfhydryl (xanthate) collectors, which were ineffective copper sulfide collectors at a pH of 12.4 and insufficiently selective at lower pH’s. The scatter of the weight recoveries at pH’s below 12.0 was partially caused by the lack of a sharp end- point in the first float. This was in contrast to the definite endpoints obtained at pH 12.3, where the froth would suddenly turn from black to white, and the pulp remaining in the cell would become a light greenish color.
Grinding and Conditioning
Attempts to float the mattes at grind times of less than 30 minutes were generally not successful. Determining if this was a result of incomplete liberation or inadequate conditioning was difficult, because conditioning with DPG occurred along with grinding. The grind after 30 minutes was 90 pct minus 500 mesh; the only coarse (plus 325-mesh) material remaining was the metallics, which tended to flatten rather than grind. Although separating liberation from conditioning was difficult, it appeared that 30 minutes was required for conditioning and that liberation occurred before conditioning was complete.
Inspection of Flotation Products
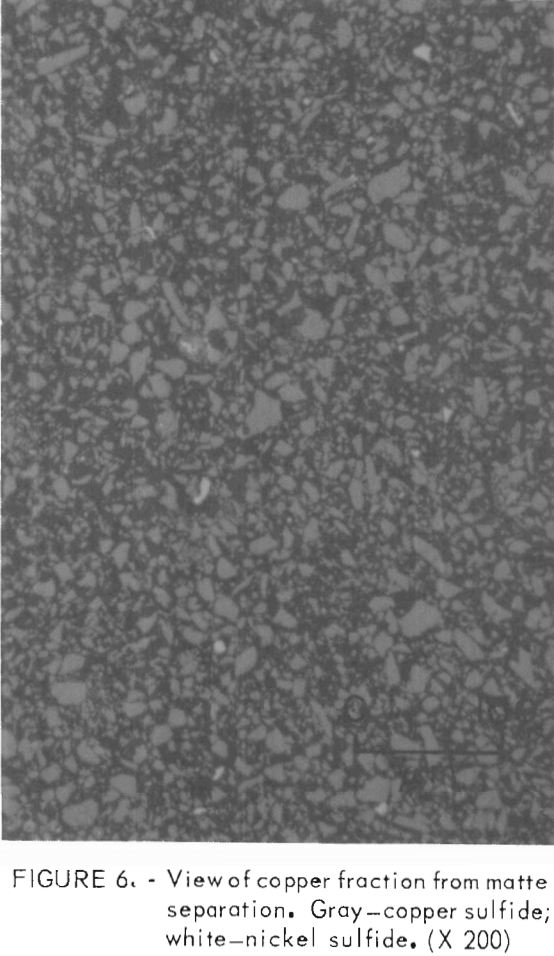
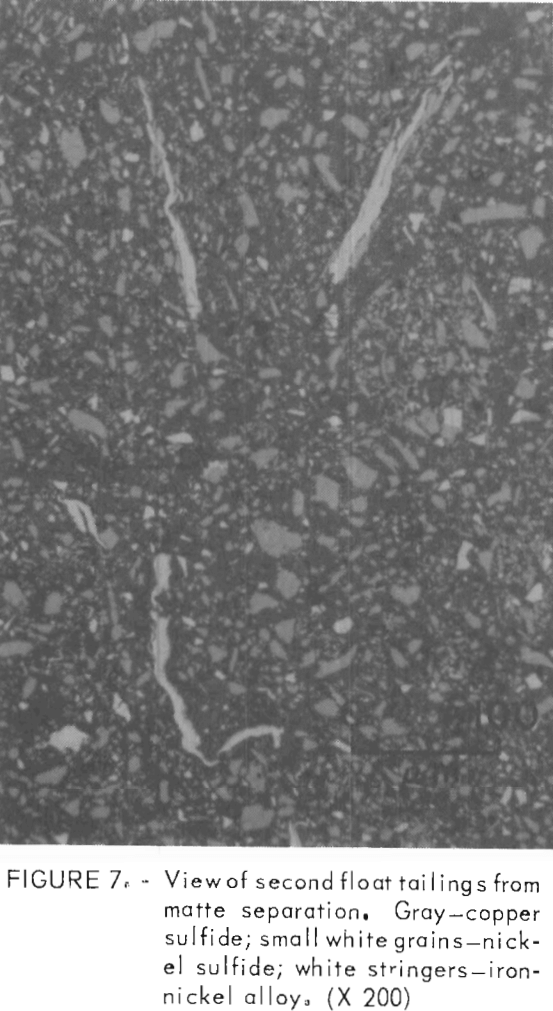
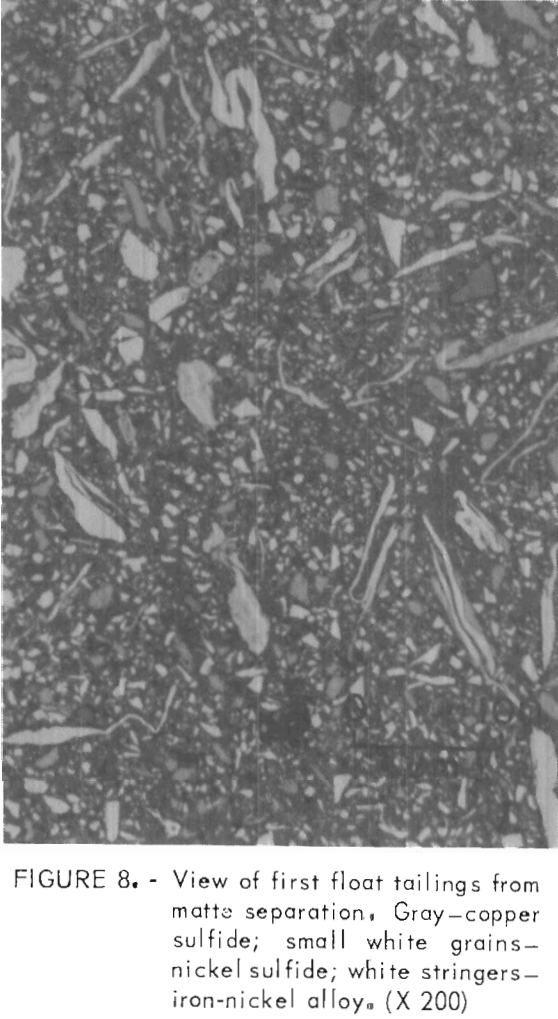
Several polished sections were prepared from the flotation fractions. Photomicrographs of these sections appear in figures 6, 7, and 8. The copper concentrate (fig. 6) is predominately chalcocite and bornite with a few scattered grains of heazlewoodite. The heazlewoodite appeared to be mostly liberated grains that were mechanically carried along in the froth. An occasional middling grain of chalcocite and heazlewoodite was also observed. The third float tailings (not shown) were similar but had somewhat larger amounts of heazlewoodite. In the second float tailings, metallic iron-nickel alloy and metallic copper flakes appeared along with chalcocite, bornite, and heazlewoodite (fig. 7). The first float tailings contained the bulk of the metallics, heazlewoodite, and some chalcocite and bornite that did not float (fig. 8).
An interesting observation was that iron-nickel alloy was frequently associated with metallic copper even though electron probe microanalyses indicated the alloy contained only very small amounts of copper. Apparently DPG was not an effective collector for copper metal or alloy, because metallics were nearly absent (approximately pct) from the copper fractions. The xanthates KEX and NIX did float a significant amount of metallics, which had to be removed from the copper fraction by magnetic separation. It was not determined if metallic copper or iron-nickel alloy was the phase floated by the xanthates. However, magnetic separation of the copper-rich fraction removed metallics high in copper (40 to 50 pct), which would indicate that copper was the predominant species being floated and that the iron-nickel alloy was mechanically attached to the copper grains.
Distribution of Cobalt and Precious Metals
The mattes contained, in addition to copper and nickel, small but significant amounts of Co, Ag, Au, Pd, Pt, and other platinum-group metals. The distribution of these metals is of interest in the planning of extraction and recovery techniques.
Cobalt was the other base metal of value in the Duluth Gabbro mattes. In sulfide systems, the chemical behavior of cobalt lies somewhere between that of iron and nickel. Therefore, cobalt would be expected to follow the nickel fractions. Averaging the results from a series of 10 float tests indicates that close to 70 pct of the cobalt content of the matte reported to the nickel fractions (table 6).
The concentration of cobalt in the copper fraction was only 0.036 pct, but the high weight recovery of this fraction meant that 30 pct of the cobalt was associated with the copper. Unless special provisions are made, the cobalt
in the copper fraction will be lost in either the subsequent converting or electrorefining processes.
Distribution of the precious and platinum-group metals in the matte separation process was determined by assaying the copper fraction, the nickel fraction minus the magnetic iron-nickel alloy, and the alloy. The magnetics were assayed separately to determine if the precious metals were concentrating in the iron- nickel alloy (table 7). The concentration ratio of the amount of gold and platinum in the magnetics to that in the nickel fraction was 10:1. The tendency of gold and platinum-group metals to concentrate in the magnetic iron-nickel alloy could be used to recover an enriched precious-metal concentrate by recycling the magnetics to the next batch of molten matte and allowing the concentration of precious metals to build up in the magnetic alloy. Currently, INCO is recirculating the copper-nickel alloy present in Sudbury mattes to accomplish this same purpose.
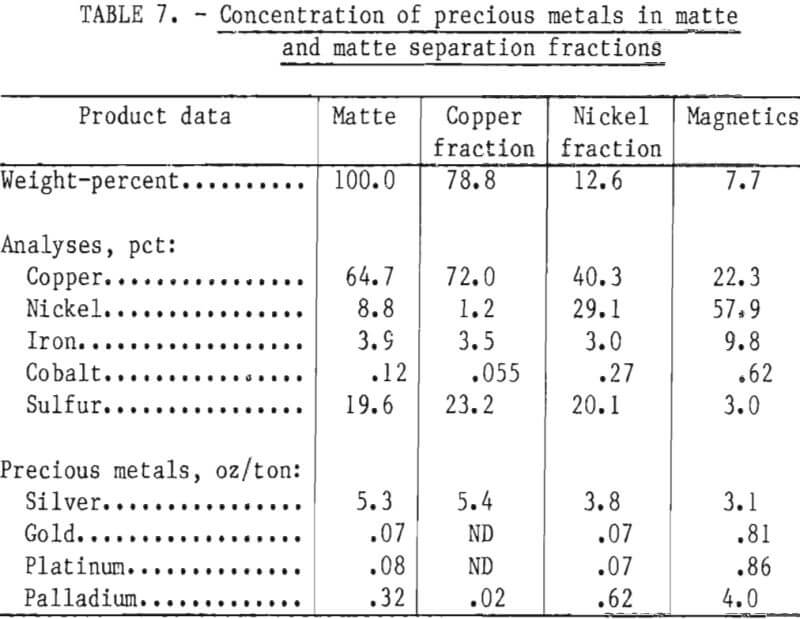
Although a small concentration of palladium was present in the copper fractions, gold and platinum were not detected. Silver tended to concentrate in the copper sulfides but not to the extent that gold and platinum did in the nickel fractions. These tendencies of silver to report predominately to the copper fractions and the platinum metals to the nickel fractions were anticipated. However, the completeness with which gold was associated with the nickel fractions was unexpected, since gold is commonly soluble in copper mattes. Apparently, platinum and gold remained in solution while copper sulfide was solidifying and were finally segregated to the iron-nickel alloy and the heazlewoodite, the sulfide with the lowest melting point in this system.
Distribution of Iron and Sulfur
Although iron is not a valuable metal in the mattes, its distribution in the matte fractions becomes important in subsequent processing. As would be expected, iron tends to concentrate in the magnetic fraction (table 7). This fraction includes the iron-nickel alloy, plus associated metallic copper and some sulfides, and analyzes about 10 pct Fe. Since the magnetics are considered part of the total nickel fraction, the end effect is to increase the iron content of the nickel fraction. This iron content must be allowed for in subsequent processing for nickel recovery.
Sulfur, in contrast to iron, tends to be concentrated in the copper fractions, which contain only minor amounts of metallics. The overall effect of sulfur distribution is to reduce the quantity of sulfur in the nickel concentrates. Since the copper fractions would likely be returned to a copper smelter, the increased sulfur could serve as additional fuel in the converting process.
Summary and Conclusions
The Duluth Gabbro matte was successfully separated into a copper fraction that could be processed through conventional copper metallurgy and a nickel fraction with a nickel-to-copper ratio of 1.
Under proper conditions (pH = 12.4, 1.7 pounds DPG per ton of matte, 30- minute grind and conditioning), 90 pct of the copper content of the mattes was recovered in a fraction containing <1.5 pct Ni and comprising 80 pct of the original matte weight. This left a nickel fraction comprising 20 pct of the original matte weight, with a nickel- to-copper ratio of from 1 to 1.3. These nickel fractions would be suitable as feed to a hydrometallurgical nickel recovery circuit. Slow cooling of the mattes beyond the normal solidification and cooling rate of the 50-pound ingots was unnecessary.
The distribution of Co, Ag, Au, Pt, and Pd was also measured to determine if recovery of these important byproducts would be feasible under the proposed processing schemes. Since 30 pct of the cobalt reported to the copper fractions, recovery problems could exist, because cobalt would be readily lost to the slag in a copper convertor.
A small amount of palladium reported to the copper fraction, and would be recovered in the electrorefining slimes, as would the silver. Recovery of the precious metals from the nickel fractions would be from the leach residues. A significant portion of the gold and platinum-group metals could be recovered as a magnetic metallic concentrate by recycling the magnetics to the nickel convertors and concentrating the precious metals in the magnetics.
The procedure developed here represents a technically feasible way to separate bulk copper-nickel mattes from the Duluth Gabbro Complex into copper-rich and nickel-rich fractions that can be treated by existing metallurgical processes.
