Table of Contents
- ARRANGEMENT & OPERATIONS
- BASE METAL LEACHING
- SEPARATING ORE FROM SOLUTION
- SOLUBILITY OF SILVER CHLORIDE IN BASE-METAL SOLUTION
- SILVER LEACHING
- PROPORTION OF SOLUTION AND ORE
- THE LEACHING TROUGHS
- SLUICE TANKS AND SLUICING
- PRECIPITATING-VAT
- CRUSHING THE LUMPS OF ROASTED ORE
- ORE-COOLING APPARATUS
- HOT-ORE FEEDING-MACHINE
- COMPLETE TROUGH-LIXIVIATION PLANTS
- ADVANTAGES OF TROUGH-LIXIVIATION
In tank-lixiviation of silver by chlorination, the extraction of the silver from chloridized ore by solutions of hyposulphite salts is performed by filtration. The ore-particles are kept stationary, while the solvent moves down through the mass of ore. The quickness of extraction, other conditions alike, is in direct proportion to the rapidity of the movement of the solvent through the ore. The solution, if left in contact with the ore without moving, displays but very little solving energy. If the filtration is interrupted for ten or twelve hours, and thus solution and ore are left in complete contact for that length of time, it will be found that, when filtration is started again, the outflowing solution is but very little more saturated with silver than it was at the time of interruption, and that the ten or twelve hours were almost a perfect loss in the total time of extraction. Notwithstanding the long contact, the solution had not become saturated with silver chloride to its full solving capacity. A rapid movement of the solvent through the ore is essential to a quick extraction. This fact is well known and the endeavor of leachers has been to hasten extraction by increasing the rate of filtration. Siphons, vacuum pumps and other devices have been used with more or less success; but none of them have given full satisfaction.
I have found that, if chloridized ore is brought into rapid contact with a proper volume of moving sodium hyposulphite solution, the silver chloride contained in the ore dissolves almost instantly, and that it is rather the volume of the solvent than its concentrated state which produces this effect.
Such favorable conditions cannot be attained in tanks. The rapidity with which a certain volume of the solvent can be brought into contact with the ore-particles is limited by the speed with which the solution descends through the ore; and thus the leaching-time in tanks cannot be shortened beyond the limit set by the filtering capacity of the ore. In a trough, however, these favorable conditions can be attained by gradually introducing the ore into the moving stream of the solvent. The ore can thus be brought into rapid contact with any desired quantity of the solvent, and moves in and with the stream. The effect is astonishing. Ore charged at the upper end of a trough of moderate length, will leave the trough at the lower end desilverized, as tailings, while all the silver chloride will be dissolved in the solution. This is accomplished during the very short time it took the pulp to rush through the trough. The shortest time with which I have experimented was three-quarters of a minute; and the extraction was complete. Further experiments may prove that with certain ores the required time for extraction will be even less. But in order to obtain satisfactory results, it is of great importance to maintain a certain proportion of solvent and ore, which proportion depends on the nature of the ore and has to be previously ascertained. Stronger solutions of sodium hyposulphite do not always lessen the required volume of the solvent. In some of my experiments I even found that I had to use much less of a one-half per cent, than of a one per cent, solution to produce the same effect.
This is in short the principle upon which trough-lixiviation is based. In the following paragraphs I give a description of the new system itself.
ARRANGEMENT & OPERATIONS
Solution is performed outside the tanks in troughs, while the ore is moving in and with the stream of solvent, and the tanks are used only to separate the solids from the liquid. The system is a continuous one; but as the lixiviation process requires two solvents, first, water for the removal of the base-metal chlorides, and then a solution of a hyposulphite salt for the extraction of the silver, it has to be divided into two departments: the base-metal and the silver department. Fig. 1 shows a complete arrangement, of which part of Fig. 21 represents the vertical section. The upper series of tanks represent the base-metal, the lower series the silver department. The tanks in each department are placed on the same level and close together. They are connected by pipes, a, b, c, d, e,f, g, h, in such a way as to form a perfect circuit. These connecting-pipes are placed a few inches below the rim of the tanks and also on a level. The diameter of these pipes depends on the daily capacity of the works, and the proportion of solvent and ore to be used. Each tank is provided with outlets, i, k, l, m, n, o, p, q, which are on a level with the connecting-pipes.  They lead to the base-metal solution trough, which is placed below the bottom-level of the tanks. Into this trough leads also the outlets, from under the filter-bottom. Each tank is also provided in the center of the bottom with a discharge-hole, through which the ore is sluiced into the silver-leach trough. The pulp (roasted ore and water) is conveyed from the furnace-house to the base-metal department in a triangular trough, which enters the building near the roof, descends in zig-zag, and branches off in two troughs which pass over the two rows of tanks. Over each tank the trough is intersected by a square box, the bottom of which contains one or more plug-holes, in order to permit the charging of any desired tank. By means of slide-gates the stream can be directed to flow in either of the two branches. The sluice troughs t, beneath the bottom of the tanks, all discharge into the silver-leach trough, which leads to the silver department. Here, the tanks are constructed and arranged in exactly the same manner as those in the base-metal department. The solution storage-vats are placed in the base-metal building.
They lead to the base-metal solution trough, which is placed below the bottom-level of the tanks. Into this trough leads also the outlets, from under the filter-bottom. Each tank is also provided in the center of the bottom with a discharge-hole, through which the ore is sluiced into the silver-leach trough. The pulp (roasted ore and water) is conveyed from the furnace-house to the base-metal department in a triangular trough, which enters the building near the roof, descends in zig-zag, and branches off in two troughs which pass over the two rows of tanks. Over each tank the trough is intersected by a square box, the bottom of which contains one or more plug-holes, in order to permit the charging of any desired tank. By means of slide-gates the stream can be directed to flow in either of the two branches. The sluice troughs t, beneath the bottom of the tanks, all discharge into the silver-leach trough, which leads to the silver department. Here, the tanks are constructed and arranged in exactly the same manner as those in the base-metal department. The solution storage-vats are placed in the base-metal building.
BASE METAL LEACHING
The pulp of water and ore, after passing a grinding-machine, in which the lumps are mashed, and of which I will speak further below, enters the triangular base-metal leach-trough, and is thus conveyed automatically to the base-metal tanks. The first tank to be charged is D; but before commencing operation, the following preparations have to be made. The slide-gate x is closed, to direct the stream into the first branch-trough, and the plug-hole of the square box above D is opened. The connecting-pipe d, between tanks D and H, is closed by a plug from the inside of D. The outlets of all the tanks are closed except the outlets n of tank H. Likewise all outlets from under the filters are closed. This done, the pulp is admitted to flow into tank D. When the solution reaches the level of the connecting-pipes, it will flow through c into tank C, and when C is filled, into tank B, and so on until the last tank H is filled, when the solution will leave the tank through outlet n, n. As soon as tank D is filled with ore, the pulp is made to flow into tank C. The connecting-pipe c is closed, and thus tank D is disconnected from the circuit. The outlet s, below the filter of tank D, is opened and the solution is allowed to drain into the base- metal solution-trough. When the solution disappears below the surface of the ore, water is admitted, to press out the solution absorbed by the ore. When this is done, sodium hyposulphite solution is applied to press out the water. As soon as the liquid flowing out through a filter-outlet s, shows indications of silver, the outlet s is closed, and the charge is ready to be sluiced for silver-leaching. While tank-charge D is under the described treatment, which does not take much time, tank C is gradually filling with ore. When filled, the pulp is made to enter tank B, and tank C is disconnected from the circuit, and its charge is treated in the same way as that of D. Charge D being sluiced out with solution, the filter-outlet s is turned from the base-metal trough into the silver-leach trough, the hose-clamp is opened, and the solution contained under the filter is allowed to flow out. This accomplished, outlet-hose s is closed and returned to the base-metal solution-trough. Then the plug of connecting-pipe d is removed and tank D is restored again to the circuit. D being empty, the flow in the base-metal solution-trough will cease until the tank is filled again with base-metal solution.
SEPARATING ORE FROM SOLUTION
In an article which appeared in the Engineering and Mining Journal, a short description of my new system of lixiviation, which brought forth some criticism. It was stated, that in order to obtain clear solutions, it would take a large number of tanks, more than double the number used in tank-lixiviation. This, however, is not the case. The physical condition of ore before and after roasting is different. When raw, a large portion of the material is in a very fine condition, which will float in water for a long time, and thus offer difficulties to separation; but when roasted with salt, the ore becomes sandy. The fine ore-particles congregate to coarser, porous grains, which behave like sand, and form but very little slimes in water. The separation takes place in a few hours. I have observed, that, while the pulp was pouring into a tank of fourteen feet in diameter, the solutions near the sides, and furthest off from the inflowing stream, became almost clear to the depth of two or three inches, which indicates a quick separation.
Across each tank, and at a right angle with the direction of the flow of the solution, there are hung six or eight curtains of coarse cloth, which reach near the bottom. They are kept in position by rocks fastened to the bottom-end, while the top-end is nailed to wooden rollers resting on the rim of the tank. The object of these curtains is to prevent the formation of streams in the tank, thus quieting the liquid, and to assist separation by the large surface which they offer, and to which the fine particles will adhere. When preparing a tank for charging, the curtains are well shaken, and rolled up to prevent them from being packed in with the ore.
The surface of the solution in all the tanks will assume the same level, and the movement of the liquid through the connecting-pipes will be gentle, and will not have the tendency of stirring up the settled material. The curtains will break the gentle current near each connecting-pipe and prevent the formation of streams in the body of the liquid, so that the flow from one tank into the other will be caused by the surface being raised by the influx. Though theoretically not quite correct, we can practically consider the out-flow to be caused by displacement. In other words, the solution which flows out from the last tank had to remain in the tanks for such, or nearly such, length of time as is required for filling all the tanks used for clearing the solution, and, therefore, a moderate number of tanks will be sufficient to obtain clear solutions.
As the chloridized ore “slimes” so very little, the quantity of slimes which will have accumulated in a tank at the time when its turn comes for charging, will be moderate. When the stream of pulp is turned in, the coarse material will go through the thin layer of slime, displace it to a great extent on the filter, and become partly mixed with it, so that the slimes will not interfere so much with filtration as might be expected. Of course, it will not be quite as free as if the ore had been charged in the usual way; but the use of the filter is so limited in trough-lixiviation, that a less rapid filtration is no obstacle, especially as in case of need a vacuum-pump can be used.
SOLUBILITY OF SILVER CHLORIDE IN BASE-METAL SOLUTION
Silver chloride dissolves in solutions of metal chlorides, especially if concentrated and hot. Solutions of moderate concentration, however, do not dissolve any, at least, not in so short a time as is required in trough-lixiviation. I made the following laboratory test: Freshly prepared silver chloride was introduced into solutions of sodium chloride which had a temperature of 120° F., and was left in contact for five minutes, during which time the solution was vigorously agitated, and then filtered. The filtrate was tested for silver with sodium polysulphide. I found :

The filtrate showed a faint coloring by sodium sulphide. When using an 8 per cent, solution the coloring was still very faint. Molten silver chloride poured into a 5 per cent, sodium chloride solution at 100° F., decrepitated into a fine powder; but the filtrate did not show any reaction for silver.
In trough-lixiviation we can regulate the grade of concentration of the resulting base-metal solution at will; and it would, therefore, be an easy matter to produce at once a solution which is suffi¬ciently dilute not to dissolve silver chloride. But the effect is different if chloridized ore is dropped into water. Some silver will dissolve, even if the resulting solution is sufficiently dilute not to dissolve silver chloride as such. It seems that by introducing the ore into water, that part of the water which penetrates the ore-particles first becomes sufficiently saturated with metal chlorides to dissolve some silver chloride. This, however, cannot remain dissolved if the resulting solution is sufficiently dilute, and is precipitated again. Therefore, by producing at once a sufficiently dilute base-metal solution, we will have some silver chloride suspended in it. The rapid motion of the pulp in the trough and its intersections is equal to a vigorous shaking in a flask, and will greatly assist in collecting the finely-divided silver into flakes. These flakes will settle together with the slimes, and are extracted by the subsequent silver- leaching. Should it be found in some cases, that the out-flowing base-metal solution still contains some finely-divided silver, it can be conveyed to a large pit outside the mill, where the small balance can settle. It is evident that trough-lixiviation offers the means of regaining the main part of the silver dissolved by the wash-water, without subjecting the latter to a separate treatment.
Should it be desired to produce more concentrated solutions (which may be the case if the roasted ore contains enough copper chloride to make its saving an object), nothing prevents the operator from producing such solutions and proceeding afterwards in the usual way.
If concentrated base-metal solutions are produced, not all of the base-metal chlorides will be dissolved, and an additional slight washing of the tank-charge will be necessary. My experiments in this direction are not complete enough to form a positive opinion; but it seems that the perfect and quick dissolving of the base-metal chlorides in water is subject to the same principle as the dissolving of silver chloride in a solution of hyposulphite salt, viz.: that a certain volume of the solvent is required to produce the effect. I made some tests in the laboratory, one with ore from the Cusihuiriachic mine, and one with ore from the San Francisco del Oro mine of Santa Barbara, Chihuahua, Mexico. The ore from the latter mine contained over 23 per cent, zinc-blende, besides some galena and iron pyrites. Both ores were roasted with 8 per cent, of salt. Twenty grammes of each were placed on a paper filter and leached with water. When all the soluble base-metal chlorides had been extracted, the filtrate was weighed, and it showed that the amount of water required for the Cusihuiriachic ore was three times the weight of the ore, while for the San Francisco del Oro it was seventeen times the weight of the ore. By treating the same quantity of ore one and a half minutes on the trough principle, no perfect extraction of the base-metal could be obtained, until the same proportion of water and ore was used as that required in common leaching, viz.: 3 to 1 for Cusihuiriachic and 17 to 1 for San Francisco del Oro. Both these ores are very base. In Cusihuiriachic it takes 9 hours to leach the base-metal chlorides of an eight-ton charge. How long it would take to leach a similar charge of the other ore I do not know, as this ore, on account of its exceedingly refractory character, has never, thus far, been treated by itself without adding other ores. But assuming the filtering property of both ores to be alike, it would follow, according to the quantity of water required, that the San Francisco del Oro ore will require 51 hours for the extraction of the base-metals by the common tank-lixiviation. While the results of these two experiments, as I said above, are not sufficient to form a conclusive opinion, they strongly indicate that in trough-lixiviation even large quantities of metal chlorides can be dissolved almost instantly, if the proper volume of water is used, an advantage which will fully be appreciated by all who have to work exceedingly refractory ores.
SILVER LEACHING
After the charge in tank D, Fig. 1, has been prepared as described above, it is sluiced out with sodium hyposulphite solution to extract the silver. The pulp enters the silver leach-trough and flows down to tank D’ of the silver department. The arrangement and construction of the tanks are the same as in the base-metal department; and when operations have been started, the connections have to be set the same as described for base-metal leaching.
By using the proper proportion of solvent and ore, the latter will drop as tailings into the tank, while all the silver chloride which can be extracted by the common chlorination-test will be dissolved by the solution. The clear solution leaving the last tank H’, flows through the silver-solution trough into the distributing-box M, and from there to any desired precipitating-tank. The bottom of the distributing-box has twice as many holes as there are precipitating-tanks. In these holes bent lead pipes are inserted from below and are fastened by flanges. Two-inch hose, of which two lead to each precipitating-tank, are attached to these pipes. The holes can be closed by long plugs. This arrangement I introduced eight years ago and have found it very convenient. The operator can direct the stream from the main working-floor, without being obliged to creep over all the precipitating-tanks, as is customary in many lixiviating-works.
When one tank is filled with tailings, it is disconnected from the circuit and the pulp is admitted into the next tank. Outlet s’, under the filter, is opened; the solution still contained in the tank is allowed to drain off, and the part retained by absorption is displaced by water. The tailings are then sluiced out with water. Where water is scarce, the wash-water can be used for this purpose.
PROPORTION OF SOLUTION AND ORE
The quantity of solution to be used for a given weight of ore depends on the nature of the ore and has to be ascertained by previous experiments. Lead sulphate diminishes the solving energy of the solution for silver chloride, and therefore on account of this and some other cause, ore containing lead will require the most, while ores free from lead, but containing copper will require the least quantity of solution, because cuprous chloride increases the solving energy of the solution. In most cases the proportion of 10 weights of solution to one of ore will be sufficient. Ore from the Yedras Mine can be successfully treated with a proportion of 3 to 1. The right proportion can easily be ascertained by constructing a temporary triangular trough, about 150 feet in length, with ¾-inch fall to the foot. The upper end has to be connected with a mixing-box 18 x 24 inches, into which a small tank of a few cubic feet discharges. The mixing-box has a partition 4 inches high and about 6 inches back from that side which contains the outlet into the trough, in order to prevent the ore from being washed down in a mass. The small tank is filled with solution to a certain mark, at which mark the surface has to be kept during the time of the experiment, by adding solution in the same proportion as it flows out. After having once ascertained the flow of solution per minute, the quantity of ore to be charged per minute has to be regulated according to the desired proportion. The samples have to be taken at the lower end of the trough, with a vessel large enough to hold the whole stream while the sample is taken. Time must be given the ore to settle. The clear solution is decanted, the ore placed on a filter, washed with water, dried and assayed. Before making the experiment with solution the base-metals have to be removed from the ore.
In the laboratory, experiments can be made on the principle of trough-lixiviation by introducing 20 grammes of roasted ore, which has previously been washed, into a graduated cylinder of 1000 c.c., in which is contained 100 c.c. or 200 c.c. of sodium hyposulphite solution, according to the proportion which is intended to be used. The top of the cylinder has to be tightly closed with the palm of the hand, and the cylinder brought into a horizontal position, and then oscillated in order to make the ore and solution pass quickly from one end to the other, to imitate the current in a trough. This is done for 1½ minute, then the contents of the cylinder are poured into a filter, washed with water to displace the silver-solution from the sand and paper, dried and assayed.
Having by experiment found the required proportion of solution and ore, the size of pumps, pipes, outlets, etc., can be calculated. Similar experiments have to be made with regard to base-metal leaching. It is also of importance to ascertain the most suitable strength of the solution.
THE LEACHING TROUGHS
A triangular shape of the troughs is preferable, because the same quantity of water will display more energy for moving the sand than on a flat bottom. An inclination of three-fourths inch per foot is sufficient. I am not prepared yet to state the required length. With one exception, my experiments have been conducted with troughs of 150 feet in length, and the extraction was always complete if the right proportion of solvent and ore had been used. Therefore I can at present only say that 150 feet will be sufficient in most cases. It did not occur to me before to try shorter troughs with the view of ascertaining the shortest length, because I naturally was of the opinion that the longer the trough the better the effect; but now I am strongly inclined to believe that much shorter troughs will be of equally good effect, at least with regard to silver-leaching, for the following reasons: At the North Mexican Mill, for want of boards I used pipes, 150 feet from the hopper or mixing-box to the first settling-tank. On account of bends and intersections it took the pulp 1¼ minute to pass through. The extraction was complete, provided the right proportion was used. At Yedras, where I was kindly permitted to make a few experiments for my own information, I had a straight trough 150 feet long and ¾ inch fall per foot. The pulp rushed through in ¾ of a minute and the extraction was complete. Now, if the trough at Yedras had bends and intersections, and thus the pulp had moved with the same velocity as in the North Mexican Mill, it would have taken but 90 feet of trough to retain the pulp ¾ of a minute, and to perform the extraction.
My first experimental trough was only 43 feet long. I tried some ore from the Cusi mine; but not knowing at that time the importance of maintaining certain proportions, I used 6 : 1, which I afterwards found not to be sufficient for this ore. The result, therefore, was not satisfactory. By maintaining the same proportion, but letting the pulp pass twice through the trough, or 86 feet, the result was exactly the same. By passing three times, or 129 feet, the extraction was only a fraction of an ounce better. This shows that 43 feet of trough had the same effect as 86 or 129 feet. I will investigate this important point at the first opportunity I have to experiment again on a large scale. If short troughs will answer, much less grade will be required. At all events, it is an easy matter to ascertain the required length of both the base-metal and the silver- leach troughs before erecting the works.
SLUICE TANKS AND SLUICING
Fig. 2 represents the vertical section, Fig. 3 the horizontal view, and Fig. 4 the front view of a settling-tank arranged for sluicing. In the centre of the bottom is the discharge-opening, 6 inches in diameter. The cast-iron discharge-tube k, of the same inside diameter, tightly fastened to the outside of the tank-bottom, corre-

sponds with the discharge-hole. The lower end of the tube is at right-angles with the upper end, and provided with flange o. The valve m, which is provided with a rubber gasket, can be pressed tightly against flange o by turning the wheel F. Flange o and valve m are made of brass. Fig. 4a shows in detail the construction of wheel F. Around the discharge-opening, and fastened to the bottom of the tank, is the wooden polygon v, in which is cut the groove p’. Around the inner periphery of the tank, and high enough to give the filter-bottom an inclination of at least ¾ inch to the foot, is the groove p. Fig. 3 illustrates the construction of the filter-bottom, which is made in section. The filter-cloth is well fastened, and kept in position by driving tightly a rope into the grooves p’ and p. The air-escape pipe d, which reaches to the rim of the tank, enters the latter close under the filter-bottom. A piece of hose is fastened to the upper end, and can be closed by a hose- clamp. In Fig. 4 q q are solution-outlets; s, filter-outlet. Connecting pipes g and h (Figs. 2 and 4) have, like the discharge-tube h, to be well coated with asphaltum varnish. In the same figures, z is the water-pipe; n the central hose, which reaches down into the discharge-tube k, where it has to remain during the process of charging. This hose ought to be very stiff. Before charging the tank the discharge-pipe is filled with water through the central hose, in order to keep the latter filled with water, which will prevent the inside of the hose from being obstructed by ore. In the base-metal department the central hose has to be connected with the water- and solution-pipe, to allow the application of either, while in the silver department they need only to be connected with the water-pipe.
When a tank is ready to be discharged, the wheel F is turned, and thus the valve m pulled back. The water is injected through the central hose, while the latter is gently moved up and down. The stream undermines the tightly-packed sand, and causes a continual caving-in, until a funnel-shaped opening is made through its depth to the surface. Then several streams are made to play on the top, while the central hose, with checked stream, is left in position to avoid obstruction of the discharge-pipe by a too sudden rush of sand.
The central position of the discharge-opening and the funnel-shape of the filter permit a quick and clean sluicing. The pulp leaving the discharge-tube enters the sluice-trough t underneath the tank, which leads to the tailings-trough u in the silver department, or to the silver-leach trough in the base-metal department. The charge being sluiced out, tank and filter have to be well rinsed, the
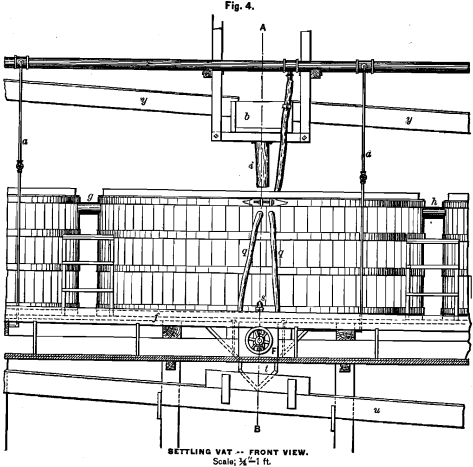



valve of branch-pipe a is opened, and the adhering sand washed off from flange o and valve m by the double sprinkler w. Then the discharge-valve is closed again, and the tank is ready to be connected with the other tanks. In Figs. 2 and 4 y represents the silver-leach trough; b, the intersecting-box above the tank, and i, a short hose made of duck.
PRECIPITATING-VAT
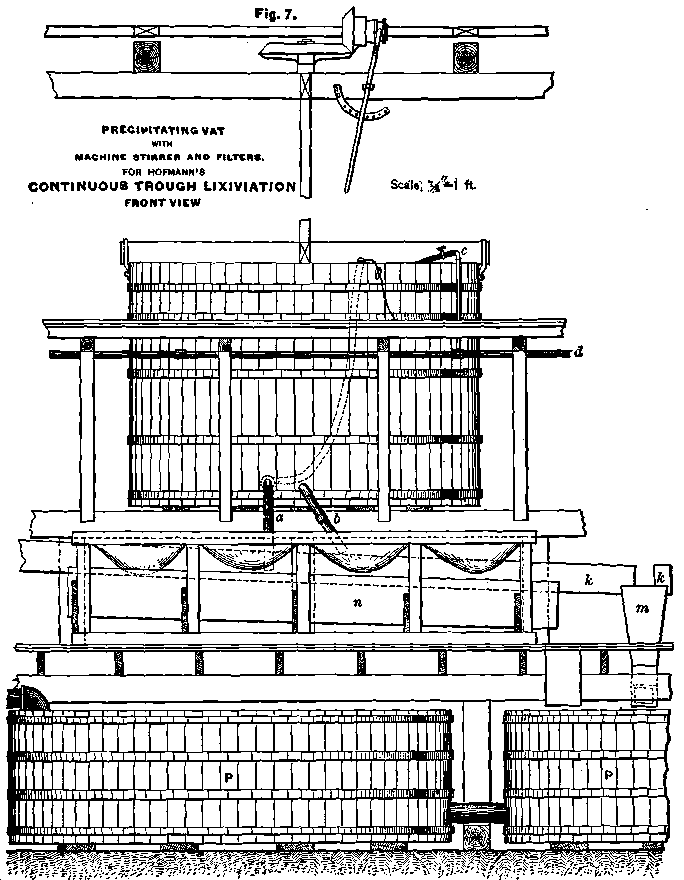
Figs. 5 to 9 illustrate the construction of my precipitating-vats, and also a very convenient arrangement of filters for the precipitate. The tanks are provided with a machine-stirrer of the construction indicated in the drawings. The stirrer s (Figs. 5 and 6) has to make about 30 revolutions per minute if the diameter of the tank is not more than 8 or 9 feet. It is set in motion, or stopped, by working the friction-clutch f (Fig. 5). The wings g (Fig. 6), which reach near to the bottom, are about 3 inches wide, and are kept in position by triangular pieces of boards. They break the violent current around the periphery and throw the solution towards the center, thus causing a strong whirling motion. In Figs. 6 and 7 a is the discharge- pipe for the clear solution, and b for the precipitate. Pipe d passes in front of all the precipitating-tanks, and conveys the calcium or sodium sulphide solution from the reservoir to the tanks. The branch-pipe c reaches above the rim of the tank, and ends in a rubber hose, which is provided with a clamp. In precipitating, the stream of calcium sulphide can be regulated, and the operator, by observing the color produced by the precipitant in the moving solution, can, after some practice, finish this operation in a very short time, and much more easily than by using buckets.
I introduced precipitating-vats with machine-stirrer first at the North Mexican mill in Cusihuiriachic, Mex., where they gave much satisfaction. One man could precipitate three tanks at a time, without assistance. The solution was so thoroughly agitated, that a very quick and perfect separation of the silver sulphide took place. The separation was so perfect that the bottom of the tank could distinctly be seen through 5 feet of solution.
In most of the leaching-works it is customary to erect only two or three very large precipitating-vats, which, in my opinion, is not favorable to precipitation. To produce a quick and perfect separation of the precipitated silver sulphide, the solution has to be vigorously agitated. This cannot be done in tanks of 14 or 16 feet in diameter. It is much preferable to have smaller tanks and a larger number of them. I construct them 8 to 9 feet in diameter and 6 feet deep. Another bad practice which I have noticed in some leaching-works is to discharge the precipitate only once a week, in some even only once a month. Fresh precipitate forms large flakes which settle easily. After two or three days it assumes a dry and sandy condition, and if stirred up, divides into very fine particles, which are kept suspended in the solution for a long time. The result of such a practice is, that the decanted solution will not be free from precipitate when used again for extraction, and a black coating of precipitate will cover the surface of the ore in the leaching tanks. This black coating is usually much poorer than the balance of the precipitate, but it is always too valuable to be lost. The precipitate ought therefore to be removed from the precipitating- tanks every day, and should never be allowed to remain longer than two days. By using smaller tanks with machine-stirrer, and by discharging the precipitate every or every other day, the circulating solution can be kept so clear, that even after a prolonged leaching of five or six days no black coating can be observed on the top of the ore.
The machine-stirrer does also good work in discharging the precipitate. After the clear solution has been decanted within a few inches of the precipitate, the stirrer is set in motion, and the discharge-pipe b opened. The stirrer agitates the precipitate until nearly all is discharged into the filters or into the vat from which the filter-press is supplied. Thus the cleaning of a tank can be done in a very short time.
Where the filter-press is not used, a filter-arrangement as shown in Figs. 7, 8 and 9, will be found very convenient. Two rows of filters are so arranged that they are in communication with each other by depressions cut into the divides of the frame. By allowing the precipitate to flow into one filter, all the adjoining filters will gradually be filled one after the other, without requiring the attention of the operator. The filters are shallow, and the precipitate will thus be spread in a comparatively thin layer over a large filtering-surface. Under each row of filters is placed a trough, n, which receives the filtrate and conveys it to the pump-tanks, P, below the floor. These filter-frames are placed in front of the precipitating-tanks and can be made to contain quite a number of filters, as shown in Fig. 1, where two sections serve for six precipitating-tanks. Owing to the shallowness of the filters and the large filtering-surface, the solution will drain off fast, and in five or six hours the precipitate will be stiff enough to be charged with a hand-shovel into the furnace.

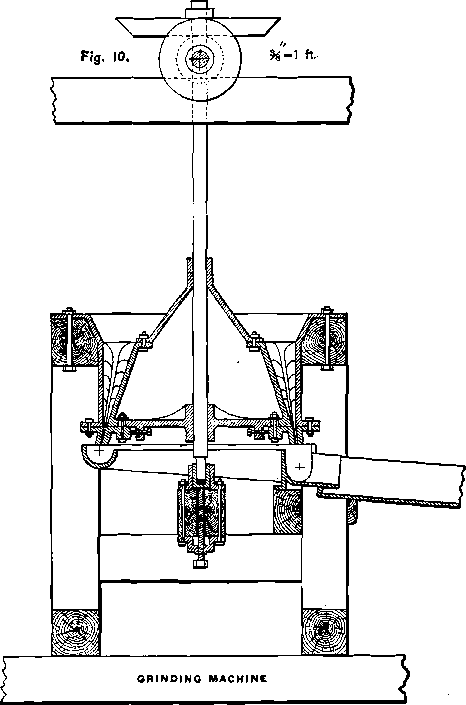

CRUSHING THE LUMPS OF ROASTED ORE
The extraction in trough-lixiviation is not produced by filtration; and the silver as well as the base-metal chlorides contained in the lamps of the roasted ore will not be extracted if the lumps are allowed to enter the trough together with the finer portion of the ore. In order to obtain good results it is necessary first to mash the lumps. An agitator will not perform the work rapidly enough, and some quick-grinding machine has to be employed for this purpose. Figs. 10 and 11 show the construction of the Kegelmuhle, which is very effective on soft material, and is used in Europe for grinding coal. It is copied from Rittinger, with some slight alterations, which I made to adapt it to trough-lixiviation. Figs. 12, 13, 14 and 15 represent another grinding-machine for soft material, which is my own device. It is based on the principle of the Kegelmuhle. The mantle m is also stationary, but, instead of being cylindrical, is cone-shaped. The muller f, a flatter cone than the mantle, is inserted from below, and can be lowered or raised by the screw g, for the purpose of regulating the fineness to which the lumps are intended to be mashed. Mantle and muller are provided with exchangeable shoes and dies. This mill has a larger working capacity than the Kegelmuhle, because the centrifugal force, created by the rotation of the muller, will greatly assist the discharge of the pulp, while in the Kegelmuhle, with an almost vertical discharge, the centrifugal force has no beneficial effect, but on the contrary, acts against the full discharge of the pulp. Towards the center, shoes and dies are provided with teeth, while towards the periphery their surface is smooth. The teeth cut the larger lumps, the smaller ones are mashed by the smooth part of the cones. Water and ore are charged through A, while the circular cast-iron trough t receives the pulp and conveys it to the base metal leach-trough o. The canvas strip k prevents the pulp from flying over the rim of the trough. The main portion of the roasted ore will be fine enough to pass through the mill without being affected, and only a small part will have to be mashed. For this reason, and on account of the softness of the wet material, large quantities of ore can be put through in twenty-four hours with but very little wear of the machine, which, in itself, is of simple and cheap construction.

ORE-COOLING APPARATUS
In trough-lixiviation, the transportation of the roasted ore to the settling tanks is effected by a stream of water, and it is apparent that by dropping the hot ore into the stream, the costly and annoying cooling-floor manipulations can be avoided. For this purpose

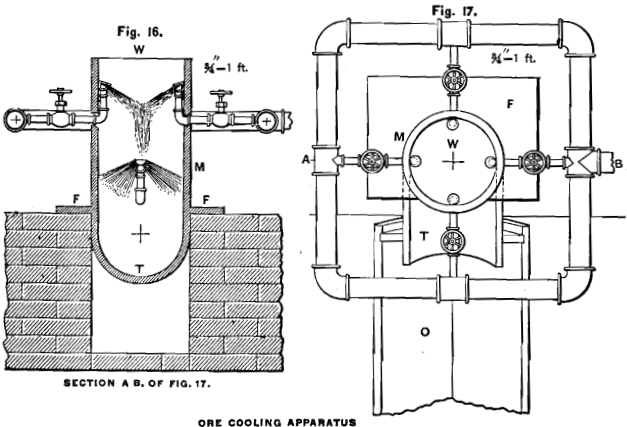
I have constructed an apparatus which is represented in Figs. 16 and 17. It consists of a cast-iron tube M with a flange or plate F to keep it in an erect position. The lower part of the tube has the shape of a trough T, and is connected with trough O, which leads to the grinding-machine. Inside the tube, and on two different levels, water-spouts are so arranged and constructed that the entering water forms sheets (not sprays) which perfectly close the tube. At the line where the two opposite sheets meet, the water will drop straight down. The sheets of water are produced by placing a smooth plate of iron properly over the obliquely-cut ends of the pipes, as shown in Fig. 16. The water-pipes inside the cooler ought to be made of cast-iron, to resist longer the action of the solution. The hot ore drops through W, and, in striking the water, is immediately carried below the sheets of water and is so quickly enwrapped in it that but little dust is formed, which will be absorbed by the sprays, while most of the steam will be condensed. The bottom T has sufficient inclination to make the ore move as soon as it strikes it.
By cooling the ore in this way, we have the additional advantage of increasing the temperature of the water used for base-metal leaching, without incurring extra expense or labor. The resulting temperature of the water will depend on its original temperature, the temperature of the ore and the proportion of water and ore used. By a laboratory-test I found that a dark red-hot ore, dropped in three times its weight of water, raises the temperature of the latter from 60° F. to 95° F.
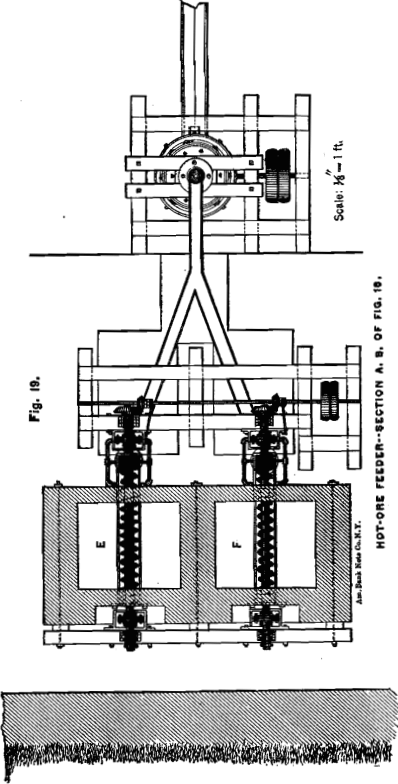
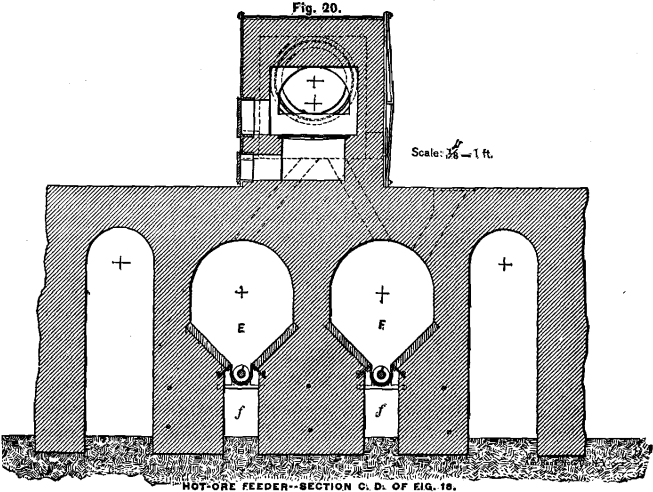
HOT-ORE FEEDING-MACHINE
In order to work the cooling-apparatus to advantage, it is necessary to charge the hot ore evenly by some mechanical contrivance. I have devised an arrangement which, in combination with a Howell roasting-furnace, is illustrated in Figs. 18, 19 and 20. The ore from the furnace drops alternately into the bins or vaults, E and F. The ore is removed from the bins by conveyer-screws. These, with the conveyer-troughs, are placed below the inclined bottom of the bins in order to keep them comparatively cool by the air in the channels, f. The 4-inch shaft of the conveyer, over which the cast-iron screw-segments are slipped, consists of three pieces which are connected close in front of the journals by large flanges, g, in such a way that the coupled ends do not touch each other. The object of these flanges is to keep the journals cool. For this purpose the vessel, h, through which a small but continuous stream of water flows, is so placed, that the flanges have to revolve through the water. The inflowing water strikes the upper half of the couplings to keep them also cool when not in motion. The conveyer discharges the ore direct into the cooling-apparatus, as shown in the diagram, whence the water carries it down to the grinding-machine.

By having, for each furnace, two bins, which are alternately filled and discharged, opportunity is given to increase the chlorination of the silver of an incompletely chloridized ore.
Not all, but most kinds of chloridized ores, when allowed to remain in a mass for some time, harden in cooling, especially on the surface and sides; and it will be found necessary in such cases to assist the discharge by poking from time to time from above. To
 facilitate this, the bin proper is only four feet deep and accessible from the top, as shown in Fig. 18. In case an ore hardens too much, and thus would make the described arrangement impracticable, the bins can be made much larger, but have to be provided each with a discharge door close to the bottom. A short conveyer has to be placed in front of the bin and right under the discharge- door. The end of the conveyer-trough nearest to the door opens in the shape of a hopper, into which the ore from the bin can be drawn by means of hoes in portions of half a ton or a ton at a time. An ore which has once hardened in cooling and has then been removed, is not likely to harden again ; and the conveyer will charge the ore evenly from this small hopper into the cooling-tube.
facilitate this, the bin proper is only four feet deep and accessible from the top, as shown in Fig. 18. In case an ore hardens too much, and thus would make the described arrangement impracticable, the bins can be made much larger, but have to be provided each with a discharge door close to the bottom. A short conveyer has to be placed in front of the bin and right under the discharge- door. The end of the conveyer-trough nearest to the door opens in the shape of a hopper, into which the ore from the bin can be drawn by means of hoes in portions of half a ton or a ton at a time. An ore which has once hardened in cooling and has then been removed, is not likely to harden again ; and the conveyer will charge the ore evenly from this small hopper into the cooling-tube.
If an ore is to be treated which roasts easily, and no increase of silver-chlorination is produced by letting the ore lie in mass, there will be only one conveyer required for each Howell furnace. The ore then will drop directly from the furnace into the conveyer- trough, and will be carried to the base-metal leaching in the same proportion as the battery supplies the furnace with raw ore. In such a case all operations, from the time the ore passes the rock-breaker, until it leaves the leaching-works as tailings, will be automatic.
When other furnaces than Howell’s are used, the roasted ore has to be dumped into bins.
I do not intend to represent the above hot-ore feeding-machines for refractory ores as perfect; but, with some manual assistance, they will perform the work, and will give much more satisfaction than the present cooling-floor manipulations.
COMPLETE TROUGH-LIXIVIATION PLANTS
If circumstances require it, trough-lixiviation can be used only for the extraction of the silver, while the base metals can be removed in the usual way. This may be necessary in case tank-lixiviation works have to be altered into trough-lixiviation works. In such cases, it will require only-a few base-metal leaching-tanks to be erected in or outside the main building, at a place which offers sufficient grade for the silver-leach trough. It is immaterial where these tanks are placed, as the connection with the silver department can easily be made by the silver-leach trough. The roasted ore will have to be elevated from the cooling-floor to the level of the base metal tanks. There are ores which retain the silver chloride tenaciously, and require a very long leaching-time. In some works five or six days, and even more, is not unusual. By changing such works into trough-lixiviation, this annoying difficulty would be entirely overcome at comparatively small expense.
If the entire works are arranged for trough-lixiviation, the advantages derived from the new system are, of course, much greater. Fig. 21 shows the complete system in connection with a Howell furnace. The leaching-works are not in the same building as the battery and furnaces, but some distance down the slope of the site, in order to gain grade. Hot-ore feeder, ore-cooling apparatus and grinding- machine are in the furnace-building. The ore, after passing through the furnace, drops into the bin, F, from which it is removed after some hours by the conveyer, and drops through the cooler. From here the water carries it through trough k, to the grinding machine. After passing through the grinder, the pulp enters the base-metal leach-trough, which conveys it down to the base-metal department, and drops into the settling-tanks, B. While the ore passes through the trough, the base metals are extracted. From here the ore is sluiced out with sodium hyposulphite solution into the silver-leach trough, and conveyed to the settling-tanks, S, in the silver-department. The extraction of the silver-chloride being performed in the trough, the sand drops into these tanks as tailings. The silver-solution flows into the precipitating-tanks, and the tailings are sluiced out into tailing-trough, t. The leaching-troughs have ¾-inch fall

per foot, with the exception of trough k, in the furnace-building, which has more (about 2¾ inches per foot), because the material to be moved in it is coarse. This trough is not triangular, like the others, but has a round bottom, in order to give the water a better opportunity to move larger lumps.
One of the objections used against trough-lixiviation has been, that a mill with this system will require more fall then an ordinary mill. Though this is true, I cannot see why this should be an obstacle to the new system. The additional grade is not needed as a sudden drop in one particular place, but is divided over the entire works; and by erecting the leaching-works further down the site, no unusual steep slope is required. As a precaution against a total destruction of the works by fire, it is even advisable not to bring all the departments under one roof, especially in this case, where the transfer of the ore from one department to the other is performed automatically and without expense.
In order to illustrate that the extra grade required does not offer any difficulties in the construction of the buildings, and that the trough-system does not require any unusually steep slope, or unusually large excavations, I have constructed the plant as represented in Figs. 1 and 21, with excessively long troughs :—base-metal leach- trough from cooler to settling-tank, E, in the base-metal department, 293 feet, and silver-leach trough from tank A (base-metal department) to tank E’ in silver-department (Fig. 1) 344 feet; in all 637 feet. It can be seen at a glance that the plant can be erected without any difficulties, and that no unusually large excavations are required. The slope of the site as represented is 22°, which I do not think an unusual grade for any mining district.
ADVANTAGES OF TROUGH-LIXIVIATION
I will now point out, in a few words, the advantages of the new system.
- The system being continuous, the operator receives for precipitation a silver solution of uniform strength in silver, which facilitates quick and exact precipitation.
- In tank-lixiviation, each tank-charge is in a different stage of the process, which requires a great deal of attention, and offers the opportunity for costly mistakes. In smaller works, this is not so much felt, but in larger works like the Cusi mill, where twenty-two leaching-tanks are in operation, and each of them in a different stage of treatment, it becomes quite complicated. Notwithstanding the trough-system requires two departments, the operations are materially simplified. In each department, attention has to be paid only to about two tanks, and the operator will have plenty of time left to attend to precipitation, etc.
- The most important advantage of trough-lixiviation is the fact that this method enables the operator to bring the ore into sudden contact with any desired quantity of the solvent. This is a very important fact, as it offers the means to overcome some annoying chemical and mechanical difficulties, hitherto encountered in the lixiviation process. The extraction of the silver from lead-bearing ores is slow, and requires an extensive plant. It is well-known to leachers of such ores that, while the main portion of the silver is extracted in a short time, the remaining few ounces will be tenaciously retained by the ore. Thus it happens that while the main portion of the silver was extracted in the first six or eight hours, the remaining eight or ten ounces of extractable silver will require three or four days, sometimes more. I have noticed cases in which only one ounce per ton in every twenty-four hours of prolonged lixiviation could be extracted. This very singular phenomenon is difficult to explain. It seems that only that part of the silver is so difficult to extract which originally was contained in the lead-mineral of the ore. I have observed that whenever the galena of the ore became richer in silver or had increased in quantity, the extraction became slow and dragging. This, together with the fact that the main portion of the silver can be quickly extracted, indicates that the slowness of the extraction is not principally due to the disadvantageous influence of lead sulphate on the dissolving energy of the solution for silver chloride, but that it must be due to some other cause which makes the silver contained in the lead-ore to dissolve only in large volumes of sodium hyposulphite solution. In troughs, I can bring the ore at once into contact with the required volume of solution, and thus produce almost instantaneous extraction. I have made experiments with some low-grade and rather “ leady ” ore from the Cusi mine:
Roasted ore…………………………….27.12 ounces silver per ton
Chlorination-test, tailings…………3.94 ounces silver per ton
By treating it on the trough-principle with different quantities of solution, I obtained the following results:

While these results show that the proportion of 18 :1 is sufficient, we can safely assume that less solution would have had the same effect, say 14:1. According to the filtering-property of the Cusi ore, it would take seventy hours to bring the ore in contact with fourteen times its weight, a leaching time which is not infrequent with ore from certain parts of the mine.
The presence of lead does not retard trough-lixiviation: it merely entails the use of larger quantities of solution, which is surely a great advantage.
The rapidity with which the silver chloride dissolves, also materially lessens the unfavorable influence of caustic lime. - This system is especially adaptable to larger works, since large quantities of ore can be worked with a comparatively small plant.
- By producing at once a sufficiently diluted base-metal solution, the small quantities of silver chloride which have dissolved will be precipitated, and will settle in the tanks; and thus the main part of it can be regained without a separate treatment.
- The system is almost entirely automatic, and offers the possibility of avoiding the costly cooling-floor manipulations which cannot be accomplished in tank-lixiviation. As, besides this, neither the charging nor the discharging of the tanks has to be done by manual labor, it is apparent that trough-lixiviation saves a great deal of manual labor without substituting for it much machinery, and thus reduces materially the current expenses. Even large trough-lixiviation works can be operated with only a few hands, an advantage which will be greatly appreciated by managers of mining enterprises in Mexico, where the inconstancy of the common laborer causes irregularity and great annoyance.
