Table of Contents
Metallic displacement-type silver recovery units are economical and practical for use by either large- or small-scale photographic processors.
Most photographic processors could significantly increase their income by utilizing a unit of this type. The large-scale processor may find it advantageous to use the metallic displacement-type unit as a scavenger following an electrolytic cell. Electrolytic units have the advantage of direct production of high-grade silver and may permit reuse of solutions, but are relatively expensive and in many instances are inefficient if used in continuous flow operation.
No significant problem was encountered in smelting the sludges to produce over 990 fine silver bullion.
For many years industrial consumption of silver in the United States has far exceeded production of newly mined silver. In 1965 the domestic industry and arts consumed 137 million troy ounces of silver, while less than 40 million ounces of new silver was produced from domestic mines. The deficit was met by net noncoinage imports, reuse of secondary silver, and by sale of U.S. Treasury stocks.
The photographic industry consumed 45 million ounces of silver in 1965, of which probably less than 25 percent was reclaimed. Image formation on photographic films and papers requires approximately 20 percent of the silver in the photographic emulsion. The remainder is dissolved by the thiosulfate fixing bath or “hypo” solution, from which it can be reclaimed. Most large commercial photographic processors and medical X-ray facilities now use some form of silver recovery system. Electrolytic cells are the most common type of recovery units used in larger installations. These permit reuse of solutions and make a relatively pure product for market, but high initial cost precludes their use in small-scale operations. Loss of most of the silver that is not reclaimed from waste solutions may be attributed to (1) small photographic firms that do not realize the possible monetary benefits from recovering the silver or are not aware of low-investment recovery methods, (2) amateur photographers who process their own films and papers, and (3) inefficiency of many presently used electrolytic recovery systems that generally recover less than 70 percent of the silver when operated in a system with continuous flow of solution through the cell.
This report describes investigations by the Bureau of Mines leading to the design and evaluation of an economical and efficient metallic displacement-type unit for recovering silver from waste photographic fixing solutions. The unit, constructed from easily available steel wool or steel window screen packed in a convenient cartridge, is applicable to any size operation and is well adapted to supplement electrolytic-type units. Solutions treated by the displacement method are not suitable for reuse. The silver is collected as a sludge composed of a mixture of silver, sulfur, and unused metallic packing.
Several patents have been issued covering the metallic filament silver recovery method; consequently, the present investigation was focused on devising simple practical means for applying the process and presenting data describing the effects of several variables on the silver precipitating efficiency and capacity of metallic packings. A smelting process for the recovery of pure silver from the precipitates (sludges) produced by metallic displacement also was devised and tested.
A batch process for the recovery of silver from used fixer by precipitation with zinc dust is described elsewhere
Potential Recoverable Silver
Although several variables may affect the potential quantity of silver recoverable from waste photographic solutions, a gallon of fixing bath from X-ray processors will contain about 1.2 troy ounces of silver; most other film fixing baths will contain from 0.4 to 0.6 troy ounce, and paper fixing baths will contain from 0.05 to 0.3 troy ounce. On the basis of the amount of film or paper processed, approximately 1 troy ounce of silver can be recovered from hypo solution derived from (1) 8,000 square inches of X-ray film (thirty-four 14-inch by 17-inch films); (2) 16,000 square inches of other black and white film (210 rolls of size 620 film); or (3) 64,000 square inches of black and white paper (800 8-inch by 10-inch prints).
During the early stages of the investigation a monitoring program was conducted in cooperation with various local medical X-ray facilities and commercial photographic processors. Most of the installations utilized electrolytic cells to treat the effluent from automatic processors. In most of the X-ray facilities, effluent from the processor flows continuously through the electrolytic cell and then to waste. In one installation, solution from the X-ray processor was accumulated in 10-gallon batches, electrolysed as a batch, and then discarded. The photographic processors generally recycled solution continuously through the electrolytic cell and back to the processor, thus maintaining a nearly constant silver concentration in the processor and cell. Constant replenishment and bleed-off of solution was used to prevent buildup of impurities.
An extended period of solution monitoring at the X-ray Department of the University of Utah Medical Center showed that approximately 1,800 troy ounces of silver per year are dissolved. Average efficiency of the electrolytic cell during an extended period was 70 percent; consequently, an improved recovery system could increase silver production in this single installation by up to 540 troy ounces per year. Monitoring tests at other X-ray facilities indicated that the above recovery is typical of those using electrolytic cells to treat a continuously flowing solution. Batch electrolysis was much more efficient with recoveries in excess of 95 percent.
A commercial custom photographic processor using an electrolytic cell to recover silver from a continuously cycling color film fixing bath recovered approximately 100 ounces of silver during a 3-week period while processing approximately 18,000 rolls of 620 or similar size film. Silver concentration in the solution was nearly constant at 3.5 grams of silver per liter (0.43 ounce per gallon). Replenishment and bleed-off of the 3.5 grams silver per liter solution to waste at the rate of 40 ml per roll of film wasted approximately 73 ounces of potentially recoverable silver during the 3-week period. This is equivalent to an annual loss of 1,250 ounces of silver.
Description of Materials
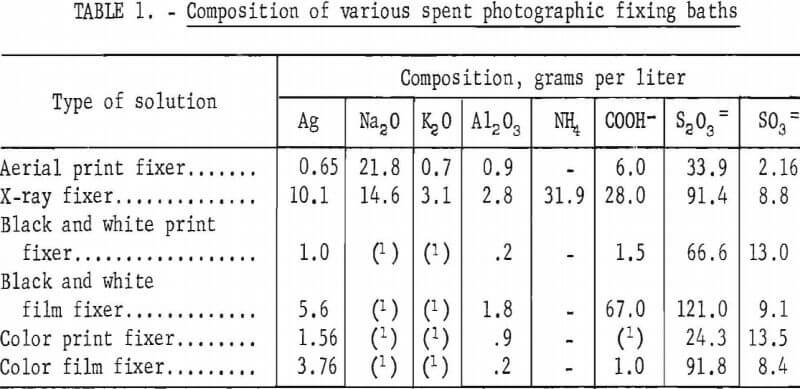
Photographic fixing solutions designed to remove unreduced silver halides from developed photographic emulsions are composed primarily of sodium or ammonium thiosulfate, which are excellent solvents for silver halides but do not dissolve metallic silver. Other constituents that may be present in varying amounts are sodium sulfite, acetic acid, boric acid, and chrome or potassium alum. The compositions of several typical exhausted fixing baths are shown in table 1. Silver and salt concentrations vary widely in different processes; however, film fixing baths are generally more concentrated than print fixing baths.
Solutions used in most of the laboratory tests were prepared synthetically by dissolving silver chloride in the following solution, which is similar to a standard commercial fixing bath:

Hereafter in the report the above solution will be referred to as the standard hypo solution.
In addition to tests using synthetic solutions, an extensive pilot program was conducted using the effluent from an electrolytic cell processing exhausted hypo from a commercial hospital X-ray processor, or in some tests the exhausted hypo was used directly as it flowed from the processor. The silver concentration in the solution from the X-ray film processor was reduced from about 10 grams per liter to 2 to 4 grams per liter by the electrolytic cell.
The metallic filaments examined were limited generally to readily available materials such as steel wool and steel window screen, which may be purchased in the ordinary hardware or department store. Zinc shavings, which are not generally available, were prepared by milling zinc rod.
Laboratory Evaluations
Iron, zinc, and copper are among the more readily available metals that are more electropositive than silver, hence will precipitate silver from solutions of silver salts. Factors that would influence the selection of the most desirable precipitant include rate of silver precipitation, cost and availability of precipitant, and precipitating capacity in weight of silver precipitated per unit weight of precipitant. The results of the tests described in the following section give comparisons of the precipitating rate of the three potential precipitants mentioned and also demonstrate the relationship between surface area and precipitation rate.
Precipitation Rate
Different forms of iron, zinc, and copper precipitants were examined to determine the effect of surface area and precipitant material on silver precipitation rate. Standard hypo solution containing 4 grams of silver per liter was tagged with a minute amount of radioactive Ag110NO3, and 50-ml portions of the solution were gently agitated with 1 gram of precipitant. Periodically samples of the solution were counted on a scintillation-type counter to determine the amount of silver precipitated. Table 2 shows the different materials used, the specific surface area per gram of precipitant, and the precipitation rate in percent per minute and in percent per minute per square centimeter of surface area.
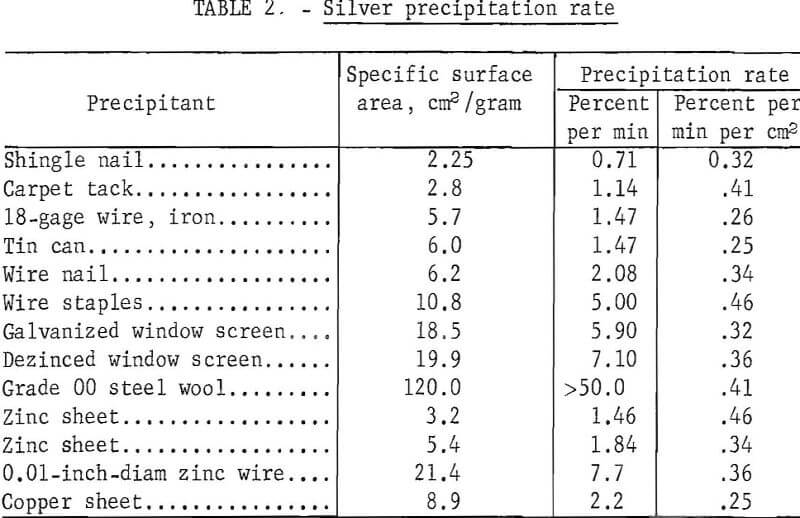
The results show that the precipitation rate is directly proportional to the specific surface area. There appeared to be no significant difference in the rate at which the different metals precipitated silver, and ensuing investigations were therefore concentrated on the cheaper and more readily available iron-based precipitants.
Effect of Barren Hypo on Metallic Precipitants
A silver recovery unit packed with any metallic filament, such as steel wool, processing a continuously flowing stream of solution would initially precipitate most of the silver near the feed inlet; consequently, packing near the outlet would be exposed to a large volume of barren hypo before it was needed to precipitate silver.
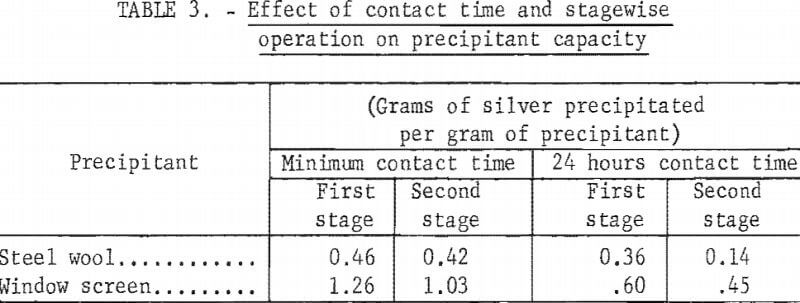
Tests were made to determine what effect contact with barren hypo had upon ensuing silver recovery efficiency of the precipitant. The test procedure consisted of agitating 1 gram of precipitant with 50 ml of radioactive Ag110-tagged standard hypo solution containing 2 grams of silver per liter for either 24 hours or the minimum time required to precipitate the maximum amount of silver. This solution, after counting on a scintillation-type counter to determine silver concentration, was advanced to another 1 gram of precipitant. At the same time, 50 ml of fresh 2-gram-silver-per-liter hypo solution was added to the first precipitant. Both mixtures were again agitated the desired length of time, radioactivity in the solutions counted, the first solution advanced, and the second solution discarded. This procedure was repeated until the first batch of precipitant ceased to precipitate silver. Thereafter, fresh hypo in 50-ml batches was added to the second batch of precipitant until it, too, ceased to function. Table 3 shows the grams of silver precipitated by each gram of precipitant. In all tests, the window screen precipitated more silver per unit weight than the steel wool. Prolonged contact of the iron with either barren or silver-bearing hypo caused oxidation of the iron and adversely affected its precipitating capacity. Increasing the contact time of steel wool with silver-bearing hypo from 10 minutes to 24 hours reduced the capacity of the steel wool from 0.46 to 0.36 gram of silver per gram of wool. Owing to the significant solubility of iron in barren acidic hypo as well as in silver-bearing hypo, preliminary contact with barren hypo in the second stage reduced the precipitating capacity of the steel wool 10 percent when using the minimum contact time per batch and 60 percent when using 24 hours contact time. Similar trends were noted with window screen; however, in all cases the capacity of the window screen was higher than the steel wool. The test results show that the minimum practical time should be used to load the recovery unit in order to obtain optimum utilization of the precipitant.
Effect of Process Variables
A number of variables characteristic either of the system producing the silver-bearing hypo or of the design of the silver collector may affect the efficiency and/or capacity of the collector. Among the variables that would be characteristic of the producing system are solution flow rate, flow continuity, silver concentration in the hypo, hypo acidity, and thiosulfate concentration in the hypo. Variables characteristic of the recovery unit are coarseness of filaments and packing density in steel wool packed units, size

of collector, and type of precipitant. The following series of tests describe the effects of the variables on collector efficiency and capacity.
Equipment and Procedure
Most of the tests to determine the effect of process variables were conducted in 1.75-inch ID by 14-inch-long plastic tubes shown in figure 1. The bed of packing in these tubes was 12 inches long to allow 1 inch of free space on each end of the tube. Evaluation of window screen was done in a square cross-section tube having the same cross-sectional area and length as the cylindrical tubes. One test also was made in a 4-inch ID by 14-inch-long cylindrical tube with a concentric 1-inch OD feed tube.
Solution, fed by a solution metering pump, flowed in the bottom of the tube, through the packing and out the top. In most tests the columns were operated on an intermittent schedule of 8 hours on and 16 hours off. Solution was not drained during the off period. Periodic samples were taken to be assayed for silver and iron or zinc, depending upon the packing used. For purposes of comparison, precipitating capacity is defined as the grams of silver precipitated per gram of original packing at the time the precipitation efficiency decreased to 90 percent. In most tests, solution flow was continued until the precipitating efficiency dropped to nearly zero; consequently, the sludges contain slightly more silver than would normally be expected in practical use.
Size of Steel Wool
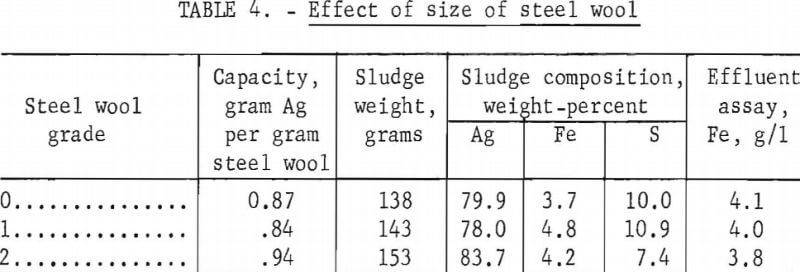
Grades 0, 1, and 2 steel wool having approximate specific surface areas of 120, 100, and 50 cm² per gram, respectively, were investigated to determine whether the size of the steel wool filaments within this range affected the capacity. Three of the plastic tubes described earlier were packed with 115 grams of each grade of steel wool and standard hypo containing 4 grams of silver per liter was passed through the column at 10 ml per minute. Table 4 shows the results of the tests. There is no significant difference between grades 0 and 1 steel wool, but the loading capacity of the coarser grade 2 wool is slightly higher than the finer grades.
Packing Density
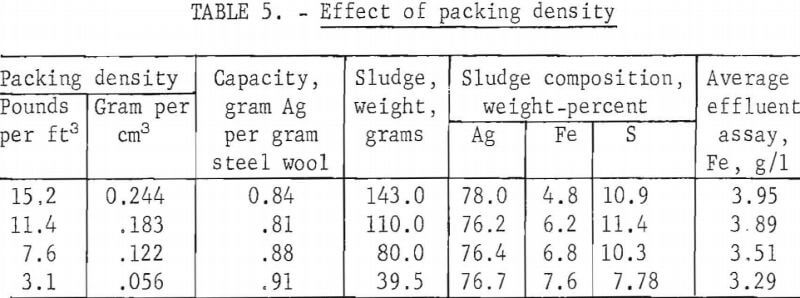
The effect of the packing density of the steel wool was determined by packing standard size columns with 115, 86.5, 57.5, and 28.8 grams of grade 1 steel wool, respectively. Standard hypo solution containing 4 grams of silver per liter was passed through the columns at 20, 15, 10, and 5 ml per minute, respectively. Test results are shown in table 5. Decreasing the packing density increased the precipitating capacity of the steel wool slightly; however, the slight increase in capacity would not warrant the additional container volume required for the looser packing.
Column Size
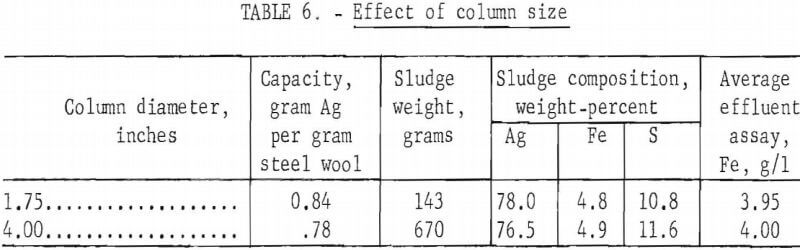
A column 4 inches ID with a concentric 1-inch OD internal feed tube was packed with a 1-foot bed of 560 grams of grade 1 steel wool. The feed tube extended to approximately 1 inch from the bottom of the column so that solution fed into the top of the tube flowed into the bottom of the column and up through the bed of steel wool. Standard 4 grams silver per liter hypo solution was passed through the column at 50 ml per minute. In table 6 results are compared with a comparable test using a smaller column. The size of the column within the range examined did not significantly affect test results.
Comparison of Different Precipitants
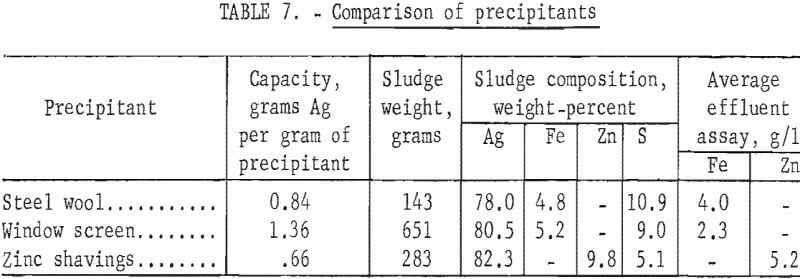
Tests were made using columns packed with (1) 362 grams of galvanized window screen, and (2) 320 grams of zinc shavings. The volumes of the columns were the same as the previous tests using 115 grams of steel wool. The window screen, cut into 1.55-inch squares, was packed in horizontal layers in a column with a square cross section. The zinc shavings were packed in a cylindrical column identical to those used in previous tests. Standard hypo solution containing 4 grams silver per liter was passed through the columns at 10 ml per minute. The results of the tests are shown in table 7, together with a similar test on steel wool. Approximately twice as much silver was precipitated by each gram of window screen than by an equal weight of zinc. Steel wool also had a slightly higher precipitating capacity than zinc.
Batch rate tests, previously described, showed that steel wool precipitated 50 percent of the silver from standard hypo in less than 1 minute. A series of tests was made to attempt to determine the maximum rate at which standard hypo solution containing 4 grams of silver per liter could be passed through a column containing 115 grams of grade 1 steel wool. Efficiency was over 99 percent at all flow rates up to 1 liter per minute through the 1.75-inch by 12-inch column. This is equivalent to 0.46 minute retention time in the fresh bed. The high efficiency was maintained until a column loading equivalent to 1.1 grams of silver per gram of original steel wool had been obtained.
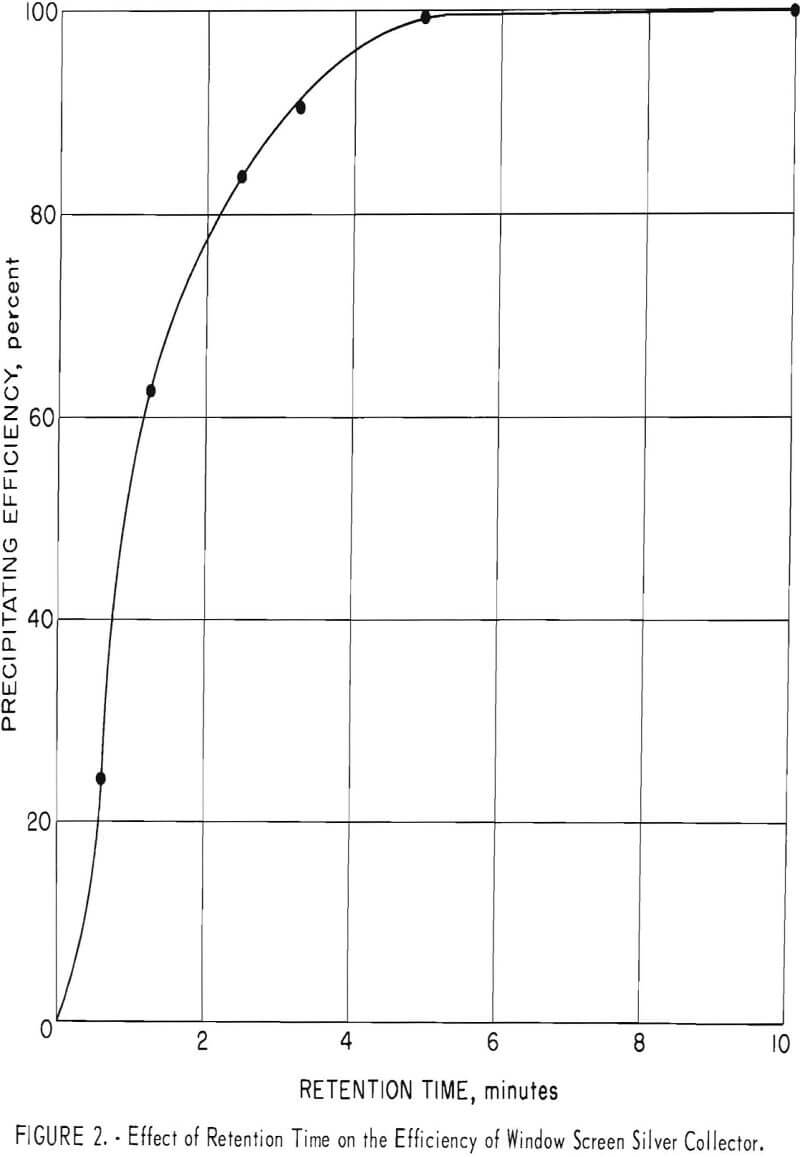
A similar test was made using the window screen-packed unit described in a previous section. Figure 2 shows the relationship between the precipitating efficiency and the retention time. The efficiency decreases rapidly as the retention time drops below 5 minutes. Although the retention time required by window screen is at least 10 times longer than that required by steel wool, practical design factors for a commercial unit could easily permit a ratio of packing volume to flow rate to provide retention times well in excess of 5 minutes.
Intermittent Operation
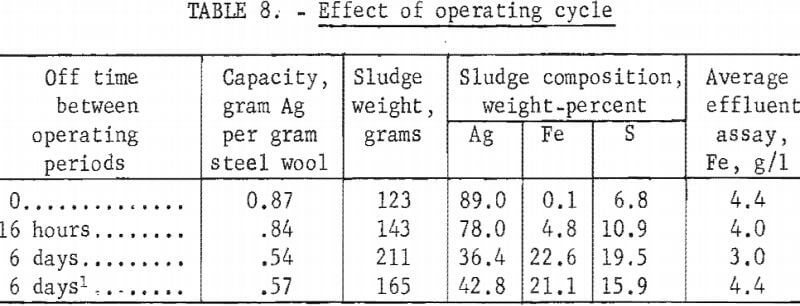
To avoid overnight operation, the normal test procedure was to operate the column for 8 hours and then stop solution flow for 16 hours leaving the column full of solution during the off period. This cycle was repeated until the precipitating efficiency dropped to less than 50 percent. The operating cycle used commercially may vary from continuous flow until the unit is loaded to intermittent operation with up to several weeks between operating periods. Tests were made to determine what effect various periods of idleness had on the precipitating capacity and sludge quality. All tests were made using columns containing 115 grams of grade 1 steel wool to precipitate from standard hypo containing 4 grams of silver per liter. The operating cycles for the tests consisted of (1) continuous operation until loaded, (2) 8 hours on and 16 hours off, (3) 8 hours on and 6 days off, and (4) 8 hours on and 6 days off with solution drained after each period of operation. Test results are shown in table 8.
Increasing the idleness period reduced the precipitating capacity and sludge quality because of increased oxidation of the iron. Draining the column between operating periods increased capacity and improved sludge quality slightly. In the design of an operating system consideration would have to be given to modifying the unit replacement cycle according to the operating cycle used.
Silver Concentration
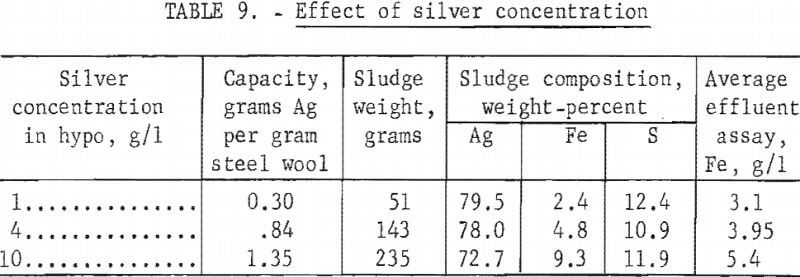
Exhausted fixing baths vary widely in silver concentration depending upon the type of photographic emulsion being processed and other processing variables. Automatic X-ray film processors generally load the fixing bath to approximately 10 grams of silver per liter, whereas solutions from black and white prints may contain less than 1 gram silver per liter. Three tests were made using columns of 115 grams of grade 1 steel wool to precipitate silver from the standard hypo solution containing 1, 4, or 10 grams of silver per liter, respectively. Table 9 shows the results of the tests.
Test results show that precipitation capacity of the steel wool increased significantly with increasing silver concentration in the hypo. Quality of the sludge decreased slightly with increasing silver concentration; however, the difference in quality would not affect subsequent refining of the silver. The consumption of steel wool per unit of silver recovered is more than four times higher for the 1-gram~per-liter silver solution than for the 10 grams per liter.
Hypo Acidity
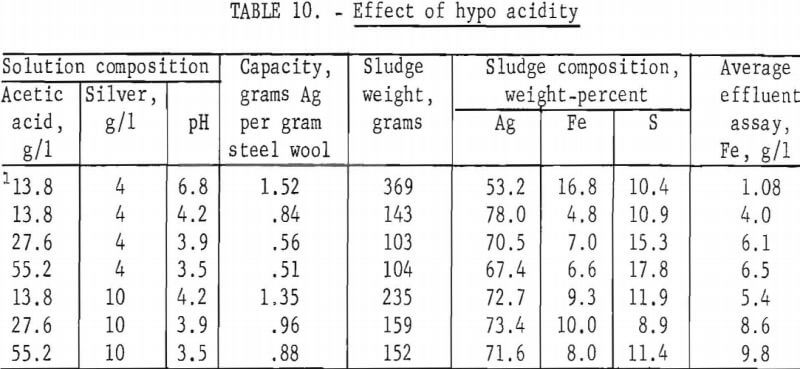
The effect of the acidity of the hypo solution was determined by adding different amounts of acetic acid to hypo solutions containing 4 and 10 grams of silver per liter, respectively. With the exception of the quantity of acid, the hypo composition was the same as the previously described standard hypo solution. The silver was precipitated by passing the solution through 115 grams of grade 1 steel wool at 10 ml per minute. Two tests were made using 4 grams silver per liter standard hypo to which 9 and 23 grams of sodium hydroxide per liter were added to increase the solution pH to 6.8 and 11, respectively. Steel wool did not precipitate silver from the pH 11 solution. Other results are shown in table 10.
Iron consumption by silver-bearing hypo involves two principal reactions:
Dissolution of the iron by the acetic acid in the hypo, as follows:
2CH3COOH + Fe = (CH3COO)2Fe + H2…………………………………..(1)
Displacement of the silver ion in the silver thiosulfate molecule with iron, as follows:
Ag2S2O3 + Fe = FeS2O3 + 2Ag………………………………………………(2)
The inability of iron to precipitate silver from an alkaline solution indicates that the displacement reaction will proceed only if the solution is corrosive to the precipitant. The test at pH 6.8 indicates that only a small amount of free acid need be present, and in this test nearly all of the iron that dissolved precipitated an equivalent quantity of silver. The stoichiometric proportions in the second reaction are 4 parts of silver to 1.04 parts of iron. Table 10 shows that 4 parts of silver was precipitated from pH 6.8 solution by 1.08 parts of iron. As the acid concentration in the solution increases, the proportion of iron dissolved by the first reaction relative to that dissolved by the second reaction also increases; consequently, iron consumption per unit weight of silver precipitated increases with increasing hypo acidity.
Thiosulfate Concentration
The effect of the concentration of the sodium thiosulfate in the hypo solution was determined by passing 4 grams silver per liter hypo containing either 60, 120, or 240 grams of sodium thiosulfate pentahydrate per liter through columns of 115 grams of grade 1 steel wool at 10 ml per minute. Results are shown in table 11. Increasing the thiosulfate concentration reduced the capacity slightly; however, the variation was too small to significantly affect design or operation characteristics.
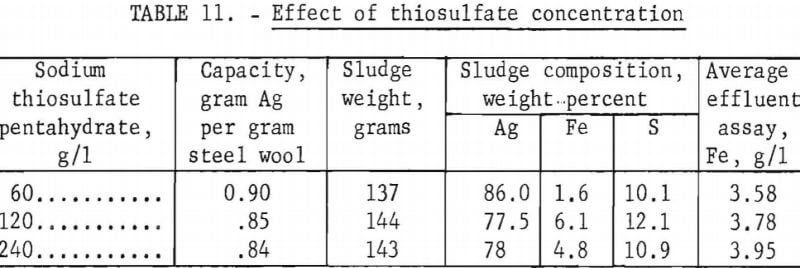
Equipment Design and Evaluation
Small-Scale Equipment
Much silver is discarded with fixing solutions produced by amateur photographers who process relatively little film during a given period. Each individual amateur photographer may not be able to practically recover his silver, but a group of amateurs may find it profitable to pool their solutions for subsequent silver recovery. These operators generally use a batch of hypo for a specified number of rolls of film and/or paper prints before discarding. Silver loading in these solutions undoubtedly varies considerably but is probably relatively low. If each gallon of hypo is used to treat 50 rolls of size 620 film, 200 8-inch by 10-inch prints, or an equivalent amount of material, the silver concentration will be approximately 2 grams per liter (0.25 troy ounce per gallon). A silver recovery system to be used in the treatment of limited volumes of solution of this nature should meet the following requirements: (1) be constructed from inexpensive, readily available materials, (2) be simple to construct and operate, (3) be efficient for precipitating silver from intermittent batches of solution, and (4) be easily storable between batches.
Figure 3 shows a unit that meets these requirements. Materials required are (1) a quart jar, (2) a plastic tee and nipple, (3) a funnel with a stem large enough to fit snugly in the tee, (4) a small cork to fit tightly in the throat of the funnel, and (5) steel wool packing. The plastic tee with nipple is inserted into the jar with the tee on the bottom and the end of the nipple level with the top of the jar. Steel wool is packed tightly around the nipple and tee. A small V-slot should be cut into the side of the cork to allow solution to flow at the rate of approximately 0.5 pint per minute.
To operate the unit, the funnel with the cork flow regulator is fitted in the pipe nipple, the unit is placed in a sink, and spent hypo is poured into the funnel. Solution is allowed to overflow the jar and drain to waste. After the available solution has passed through the unit, the funnel is removed, the jar is closed with a lid, washed off, and placed in storage until another batch of solution becomes available.
Examination of the data in table 9 shows that 1 gram of steel wool will precipitate approximately 0.4 gram of silver from standard hypo containing 2 grams of silver per liter. Examination of the data in table 8 shows that continuous operation does not give significantly different results than

overnight idleness; however, 6-day idleness between operating periods reduces capacity by about 40 percent. On the basis of the above results, steel wool recovery units should precipitate approximately 0.4 and 0.24 gram of silver per gram of steel wool when operated continuously or at weekly intervals, respectively.
Two confirmatory tests were made using jars packed with 100 grams (approximately 0.25 pound) of grade 1 steel wool to treat hypo containing 2 grams of silver per liter. Solution was passed through the units at 200 ml per minute (approximately 0.5 pint per minute). In one test, a total of 26.5 liters (7 gallons) of solution was passed through the jar at one time and in the other test 3.8 liters (1 gallon) was passed through each week for 5 weeks. Continuous operation and intermittent operation precipitated 0.41 and 0.24 gram of silver per gram of steel wool, respectively, before the efficiency dropped to 90 percent. This is in close agreement with the estimated capacity. Table 12 shows the composition of the sludges produced. The quality of the sludge from the test using continuous operation is considerably better than the sludge from intermittent operation, because the latter permits extensive oxidation of the iron during the long periods of idleness. Rapid loading of the unit is preferable when possible; however, intermittent operation is satisfactory.
A test was made using a jar packed with pieces of galvanized window screen. Efficiency dropped below 90 percent at flow rates exceeding 0.02 pint per minute. At a flow rate of 0.02 pint per minute efficiency dropped to 90 percent when 0.27 gram of silver had been precipitated by each gram of screen. On the basis of results shown in figure 2 and table 7 the permissible flow rate should have been at least 10 times greater than that used and the capacity of the screen should have been significantly greater than that actually achieved. The poor results are probably due to channeling of the solution through the packing. On the basis of these results steel wool packing is preferred over window screen in small scale units.
Large-Scale Equipment
Most large commercial photographic installations can significantly increase their silver recovery by utilizing a metallic displacement-type unit as a primary collector or as a secondary collector to supplement presently used continuous flow electrolytic-type systems. Commercially built filament units are available from local dealers and mail order firms as listed in local classified directories and the Thomas Register of American Manufacturers. The following sections describe the construction and operation of two alternative collectors, either of which gives satisfactory recovery.
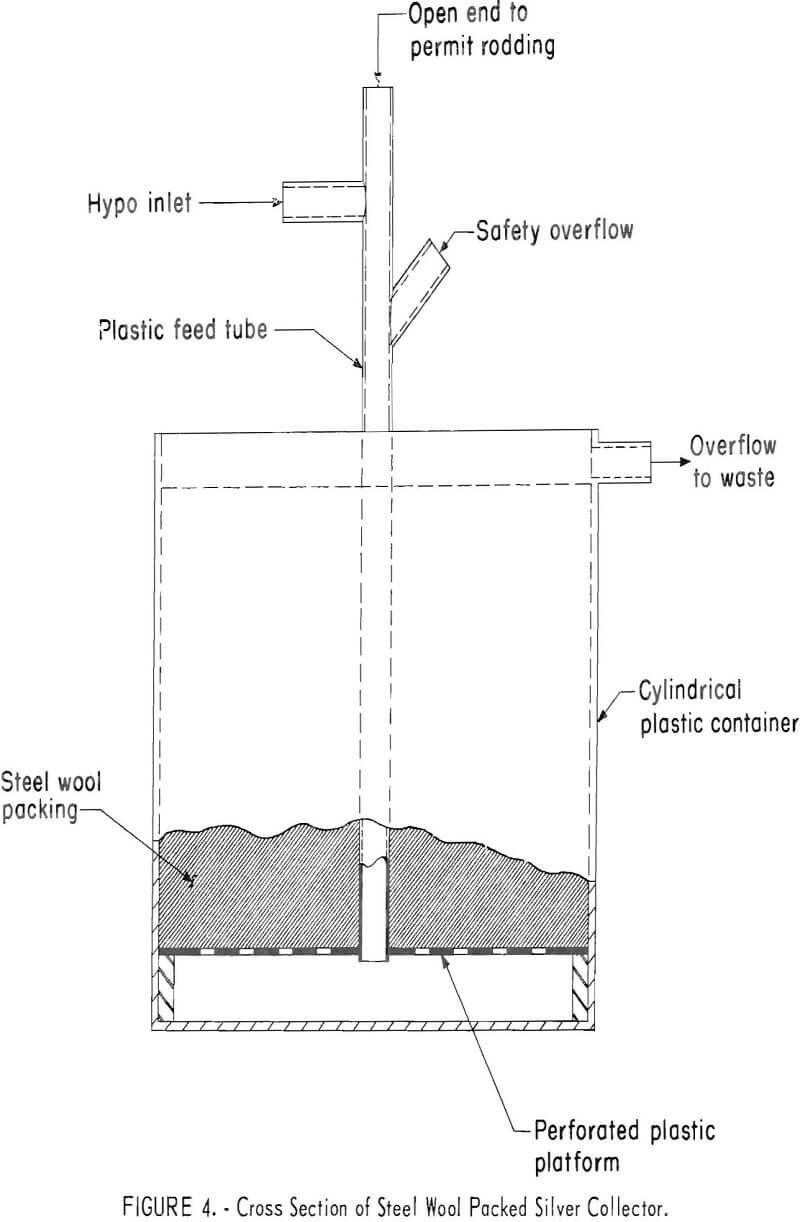
Steel Wool Packed Unit
Figure 4 is a diagram of a typical steel wool packed unit, the size of which may be varied to meet requirements. The unit used in the investigation was a 5-gallon container packed with 3,632 grams (8 pounds) of grade 1 steel wool. If construction of a unit is undertaken, the unit should either be constructed of plastic or surfaces exposed to the corrosive solutions should be protected with a suitable chemically resistant coating. The unit operated satisfactorily over a period of 7 weeks as a primary collector on a commercial-size medical X-ray processor. On one occasion collection of salts in the bottom of the feed tube plugged the unit; however, the tube was opened by rodding and no further blockage occurred.
During the initial 32 days of operation, 98.5 percent of the silver was precipitated from 500 liters (132 gallons) of solution containing an average of 9.72 grams of silver per liter (1.18 troy ounces per gallon). During the next 16 days, the efficiency gradually dropped to 85 percent. Overall recovery during 48 days of operation was 94.0 percent from 760 liters (200 gallons) averaging 9.72 grams of silver per liter. Precipitating capacity when the efficiency dropped to 90 percent was 1.7 grams of silver per gram of steel wool. This is somewhat higher than the capacity of 1.35 achieved in small-scale tests treating hypo containing 10 grams of silver per liter. The sludge from the test weighed 10.4 kilograms, and assayed 66 percent silver, 6.6 per-cent iron, and 12 percent sulfur. Reduction smelting of the sludge using a procedure described in a later section produced 6.8 kilograms (218 troy ounces) of 998 fine silver.
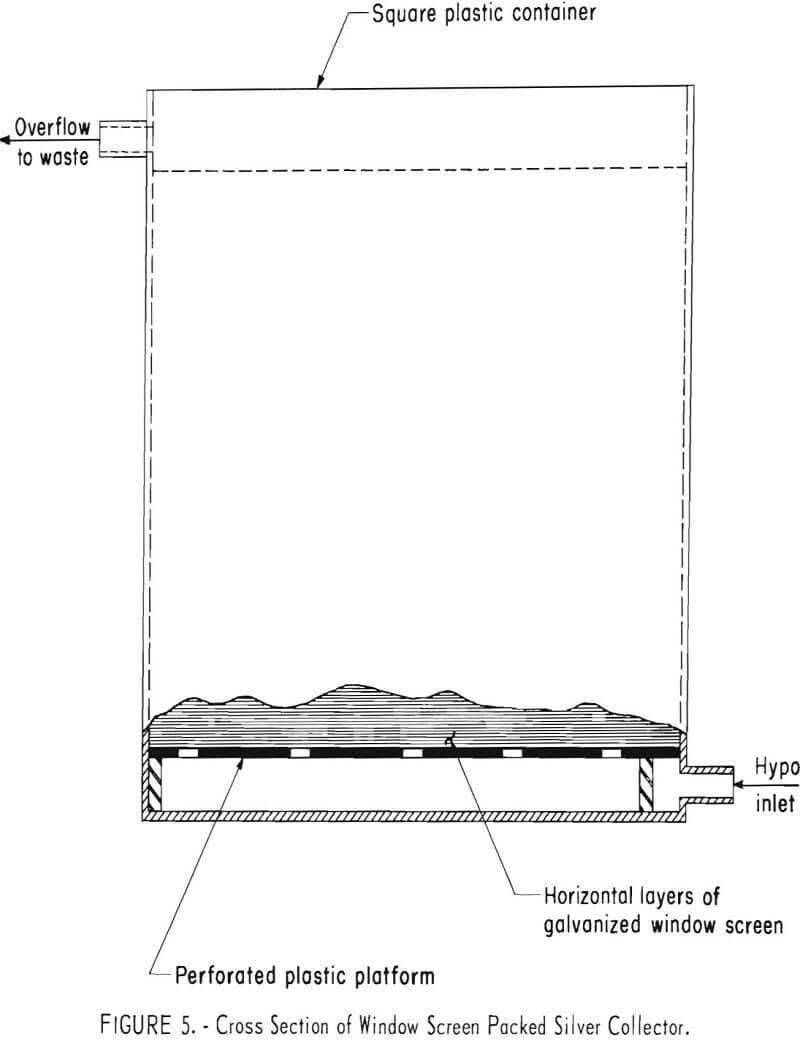
Window Screen Packed Unit
Earlier work showed that galvanized steel window screen was a suitable alternative to steel wool as a silver precipitant. Figure 5 is a diagram of such a unit. The unit containing 10,450 grams (23 pounds) of 10-inch squares of window screen arranged in horizontal layers was used initially as a scavenger on the effluent from the previously described electrolytic cell processing X-ray fixer. During a period of 3.5 months, 5,150 grams (165 troy ounces) of silver was precipitated from 1,580 liters (418 gallons) of solution containing an average concentration of 3.52 grams of silver per liter (0.43 troy ounce per gallon). This represents average recovery efficiency of 92.4 per-cent from the electrolytic cell effluent.
At the end of the initial 3.5-month operating period, the recovery unit was installed directly on the X-ray processor effluent, replacing the electrolytic cell. During 4 weeks of operation as a primary collector, an additional 3,830 grams (123 troy ounces) of silver were recovered from 393 liters (104 gallons) of solution containing an average of 10.4 grams of silver per liter (1.27 troy ounces per gallon). This is equivalent to an average recovery efficiency of 93.6 percent.
At the end of the 4-week period of operation as a primary collector, the unit was again operated as a scavenger for an additional 6-week period. During this period, 3,720 grams (120 troy ounces) of silver was precipitated from 1,265 liters (334 gallons) of solution containing an average of 3.42 grams of silver per liter (0.42 troy ounce per gallon). This represents average recovery efficiency of 86 percent during this period.
At the end of the 6-month period, the collector was removed and the sludge was dried. The dried sludge weighed 16.9 kilograms and assayed 75.1
percent silver, 4.7 percent iron, and 8.2 percent sulfur. Reduction smelting of the sludge produced 12.7 kilograms (409 troy ounces) of 999 fine silver.
Estimating When Unit is Loaded
The data presented in the previous sections can be used to estimate the capacity of a recovery unit and the volume of solution that can be efficiently treated. When the unit approaches maximum loading, silver concentration in the effluent will begin to rise. If a piece of bright copper is immersed in a hypo solution, the copper will darken if silver is present. The intensity and speed of the discoloration is roughly proportional to the concentration of the silver. If 0.1 gram of silver per liter is present, noticeable darkening of the copper occurs after 30 seconds of immersion. It would be advisable for the user to prepare his own standards by immersing pieces of copper in hypo solutions of different known silver concentrations and thus obtain first-hand observation of the relationship between silver concentration and copper discoloration.
Marketing the Sludge
Owing to assaying and handling charges, silver sludges containing less than 100 ounces of silver are difficult to market at a profit; hence, small operators may have to arrange to pool their sludges to accumulate a marketable quantity of sludge in a practical length of time.
When a marketable quantity of sludge has been accumulated, contact should be made with a smelter or purchaser of secondary silver to obtain shipping instructions, prices, and charges. Addresses of smelters or other potential buyers may be found in local classified directories or in the Thomas Register of American Manufacturers, available in most larger libraries.
Smelting the Sludge
The following sections describing smelting procedures for preparing pure silver from the sludges have been included for the benefit of individual operators who may wish to smelt their own sludge or for the benefit of commercial smelters who may not have experience with this type of material. The silver sludges produced by the iron displacement-type process may vary widely in composition, depending upon several factors described in previous sections. Smelting tests were made on two sludges which were representative of the highest and lowest grades of sludges likely to be produced. The high-grade sludge assayed, in percent, 80.5 silver, 5.2 total iron, 1.7 metallic iron, and 9.0 sulfur. The low-grade sludge assayed, in percent, 27.0 silver, 26.2 total iron, 4.4 metallic iron, and 13.8 sulfur.
Pure silver can be produced by either oxidation or reduction smelting; however, reduction smelting is preferred because of its greater adaptability to a wide range of sludge compositions without detailed analysis of the sludge.
Reduction Smelting
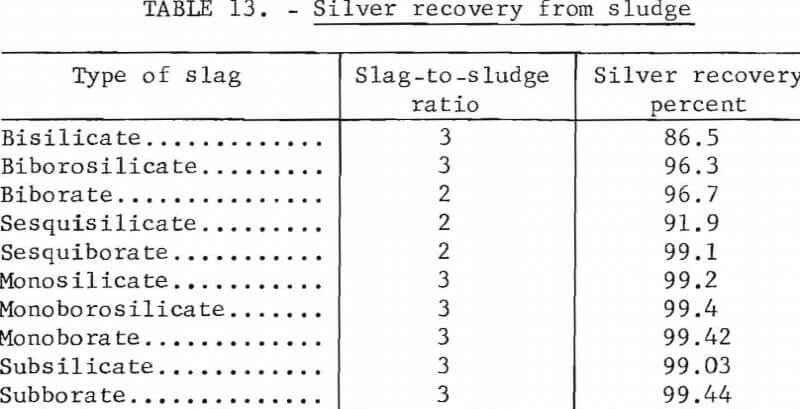
The soda-iron method was found to be the most satisfactory reduction smelting process of those tried. The procedure consists of smelting the sludge with a flux containing sodium carbonate and silica or sodium borate. A bar of iron inserted into the molten mixture provides excess metallic iron that reacts with the sulfur and forms ferrous sulfide, which is soluble in the sodium-rich slag. Residual metallic iron in the sludge is generally ineffective in providing the required reducing power. Over 990 fine (99.0 percent pure) silver was produced using slags ranging from subsilicate or borate to bisilicate or borate; however, silver recovery decreased with increasing slag acidity as shown in table 13. This tabulation is derived from tests treating high-grade sludge; however, similar recoveries and product purities were obtained from low-grade sludges using the same flux composition and flux-sludge ratio. The monoborate charge is composed of 100 parts of sludge, 218 parts of sodium carbonate, and 82 parts of anhydrous sodium borate (borax glass). The subborate charge is composed of 100 parts of sludge, 255 parts of sodium carbonate, and 44 parts of borax glass. Over 99 percent of the silver was recovered from both high- and low-grade sludges using either the monoborate or subborate slag. Smelting time requirements were not extensively investigated; however, in all tests using up to 500 grams of sludge, smelting was complete in 1 hour at 1,250° C.
Oxidation Smelting
Oxidation smelting utilizes an oxidant, usually potassium or sodium nitrate, to (1) oxidize sulfur to sulfur dioxide and alkali sulfate and (2) oxidize iron to ferrous oxide. The following equations represent the oxidation reactions:
2NaNO3 + 2S = Na2SO4 + SO2 + N2……………………………………….(3)
2NaNO3 + 5Fe = 5FeO + Na2O + N2……………………………………….(4)
14NaNO3 + 10FeS = 5Na2SO4 + 5SO2 + 10FeO + 2Na2O + 7N2…..(5)
In practice, niter is less than 75 percent effective. Investigations showed that 50 to 70 percent excess over stoichiometric was required to produce a high-grade bullion. Slag must be provided to dissolve the ferrous oxide. Bugbee states that a suitable flux mixture contains 0.33 mole of Na2B4O7 and 1.5 moles of SiO2 per mole of total iron. Sodium carbonate is added to provide sufficient basic oxygen to reduce the molar ratio of acidic oxygen to basic oxygen to 1.5 to 2. In general, acid oxygen is that oxygen present in nonmetallic oxides such as SiO2 and B4O6 while basic oxygen is that oxygen present in metallic oxides such as Na2O and FeO. Table 14 shows reagent requirements for smelting the sludges described earlier. Sodium nitrate requirements shown are 60 percent in excess of stoichiometric requirements. Slag constituents are based on an acidic oxygen to basic oxygen molar ratio of 1.5.
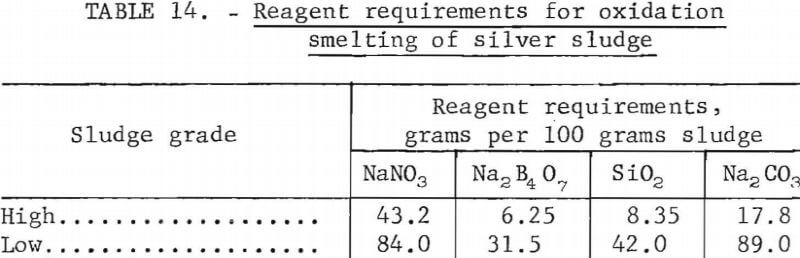
Smelting the sludges with the reagents shown in table 14 at 1,250° C for 1 hour in fire clay crucibles gave 990 fine silver. Silver recovery from the high-grade and low-grade sludges was 98.9 and 97.6 percent, respectively. The crucibles were not severely attacked by the reaction mixture.
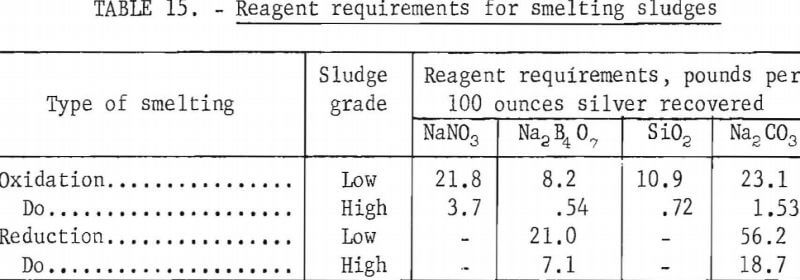
Table 15 shows reagent requirements for the recovery of 100 troy ounces of silver from the high- and low-grade sludges using oxidation and reduction smelting.