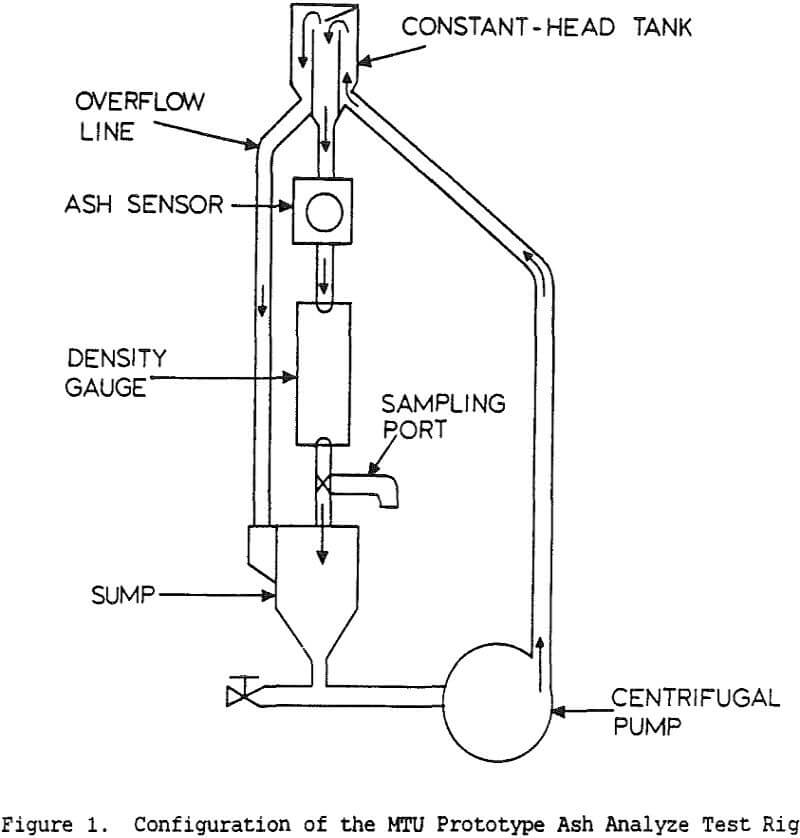
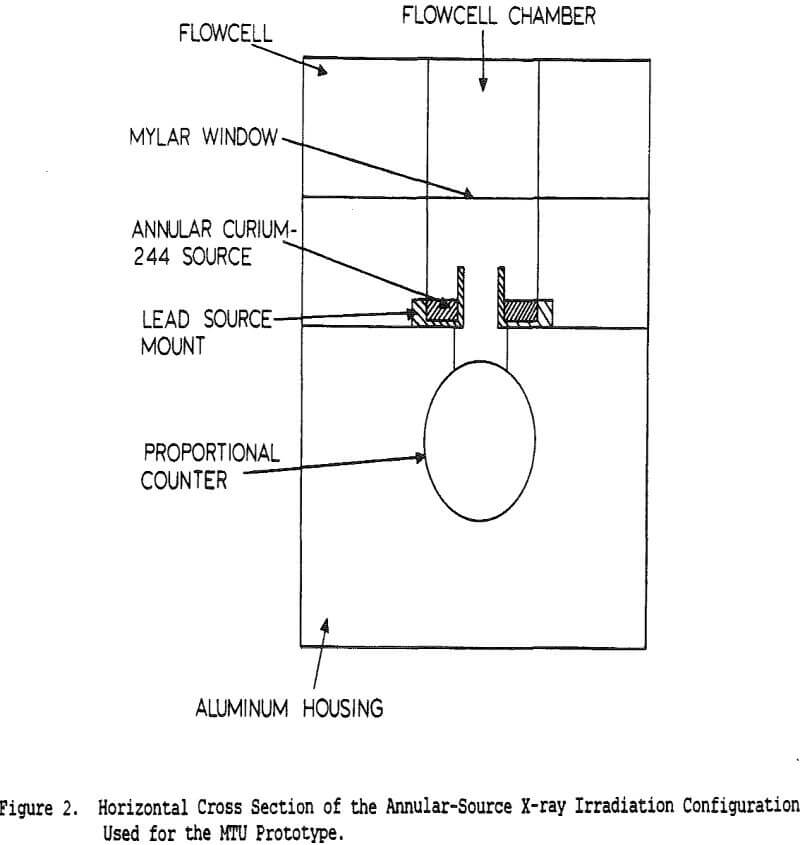
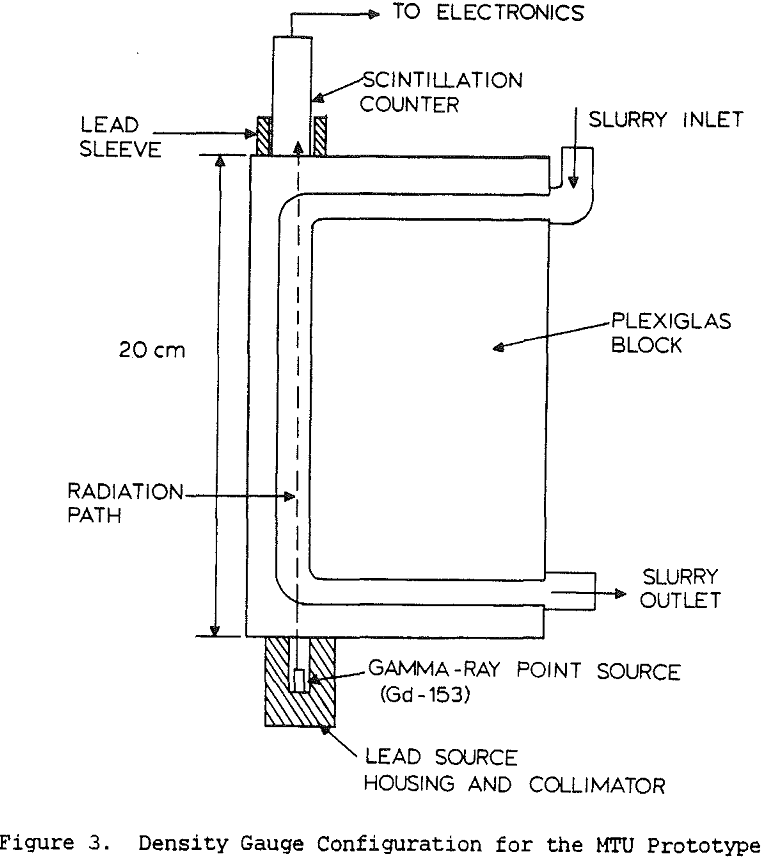
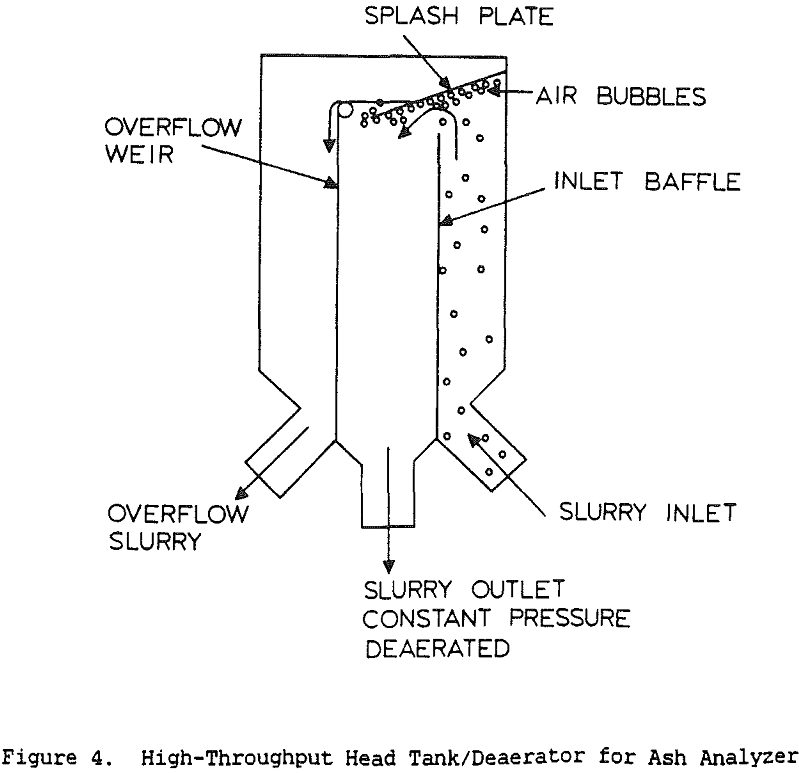
Table of Contents
Coal cleaning plants have traditionally been operated with the primary goal of minimizing the operating cost, with little attention to quality control. However, increasingly stringent air-quality regulations have made the product quality more critical. Without automatic controls, plants often must sacrifice recovery in order to meet specifications. Instrumentation and control, by providing a means for continuously monitoring and adjusting product quality, allows the product to be held within specifications while maximizing the recovery at that grade.
Theory of Operation
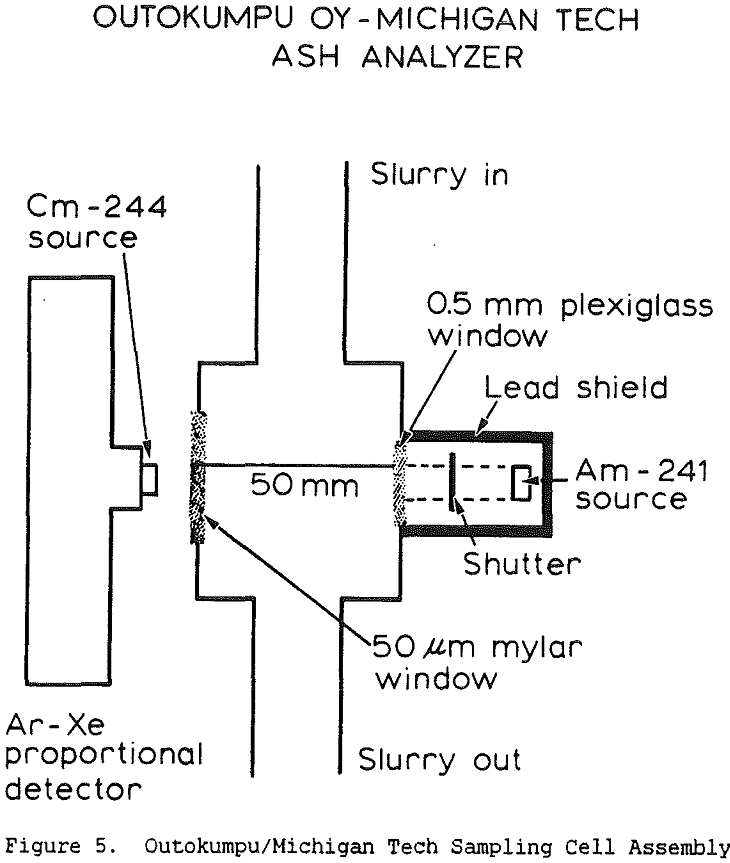
The composition of a coal slurry can be simplified to four components; water, carbonaceous material, low-atomic-number minerals, and high-atomic-number minerals. Since these must add up to 100%, there are three independent unknowns and therefore three different measurements must be made. The measurements used in this instrument are y-ray absorption, x-ray scattering, and x-ray fluorescence.
Slurry density strongly affects y-ray absorption, with increasing density producing increased absorption. For non-coal applications, it is usual to use y-ray energies of 0.7 to 1.2 MeV, since at higher energies the absorption is less affected by atomic number and is therefore more directly related to the slurry density. However increasing the photon energy also reduces the sensitivity of the measurement, so for coal slurries it is best to keep the energy below 1 MeV since the specific gravity of the slurry never becomes very high.
Coal ash is the oxidized residue remaining after coal has been burnt and consists essentially of alumina, silica, iron minerals and compounds and small quantities of the oxides of potassium, calcium, titanium, etc. In the coal, the effective atomic number of ash is about 10, while that of combustible carbonaceous material is about 6. Thus, since the effective atomic number of coal increases with increasing ash content, the ash content can be theoretically determined by measuring the intensity of scattered low energy photons.
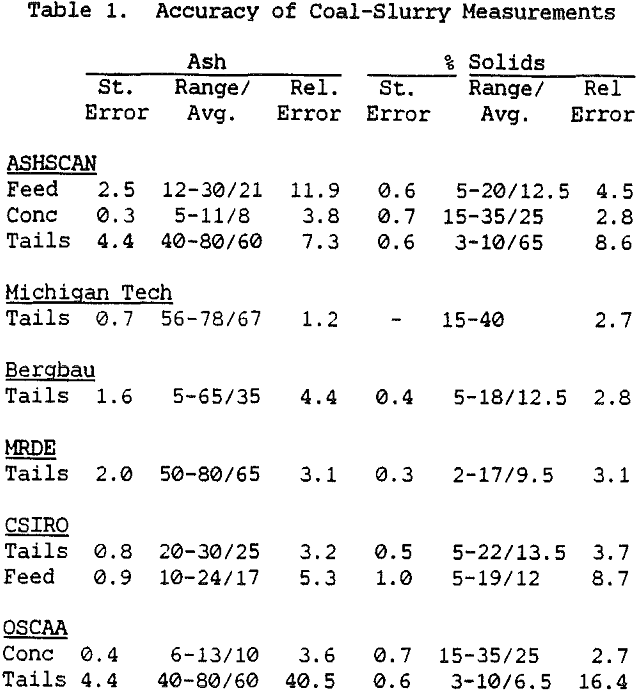
The primary use of these instruments has been to measure the ash content of slurry streams which are fairly high in ash, such as the feed and tailings streams. In normal coal cleaning circuits, this is sufficient for maintaining coal quality. However, greater sensitivity is needed if the ash content of the clean coal is to be measured and tightly controlled, as would be necessary in a plant for producing
Experimental Procedure and Results
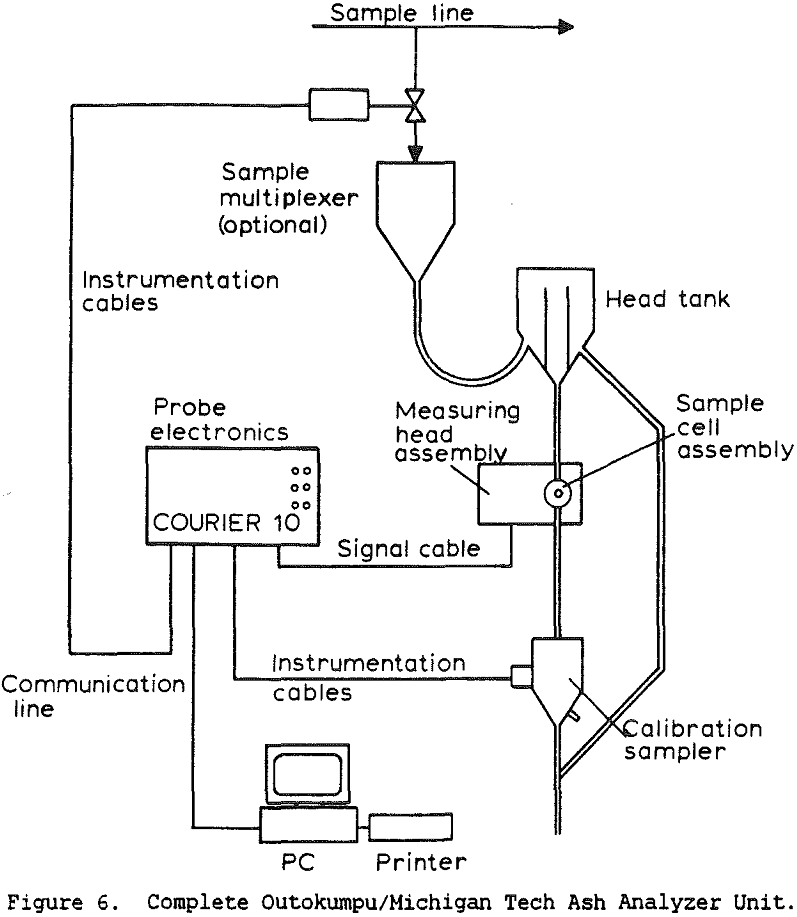
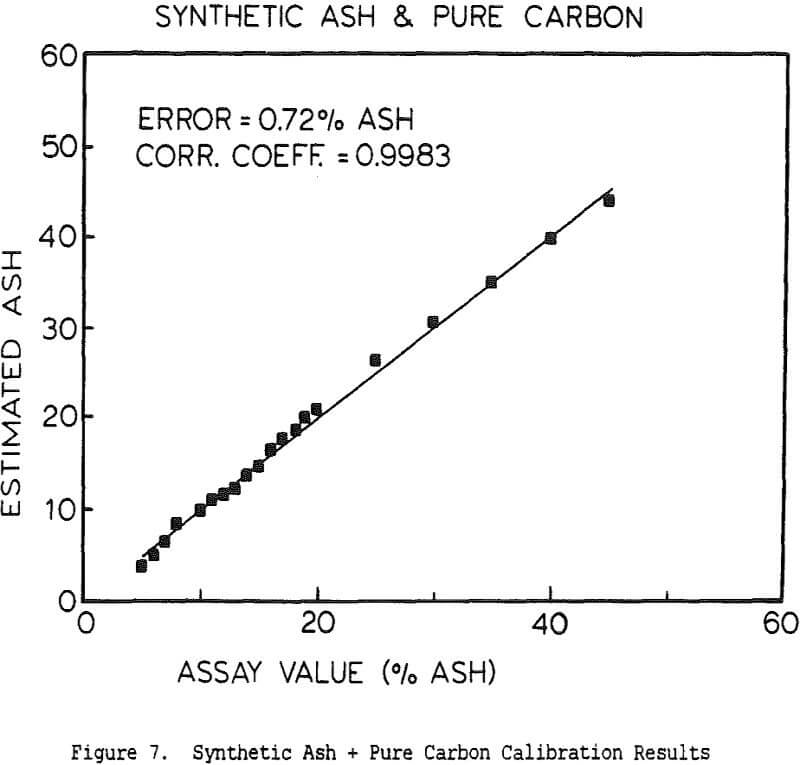
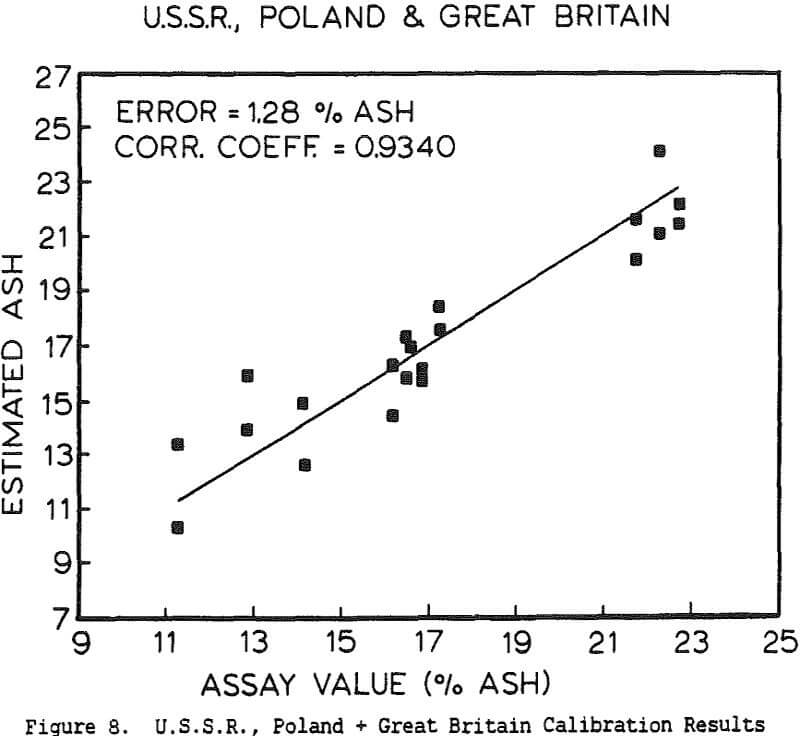
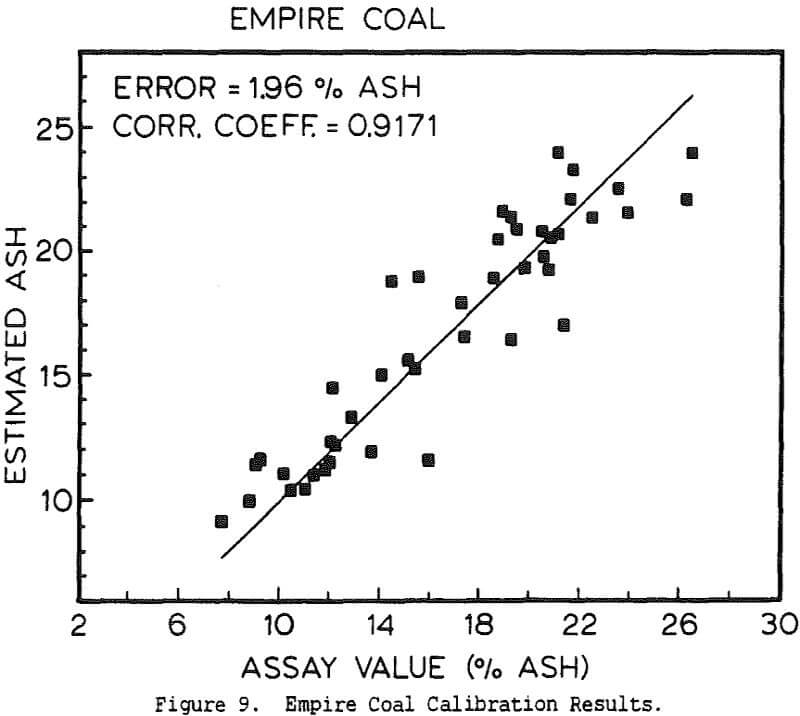
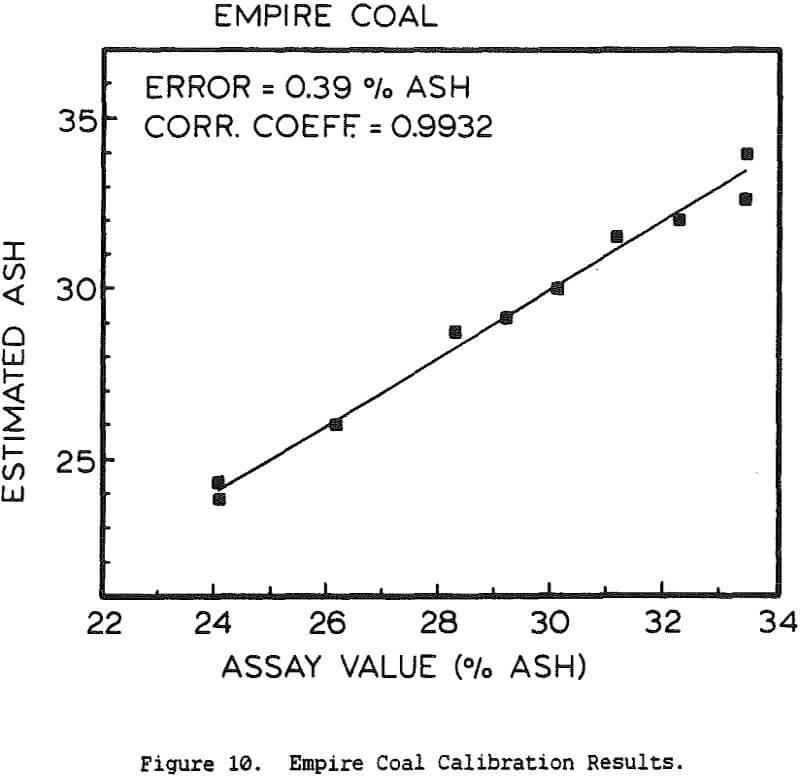
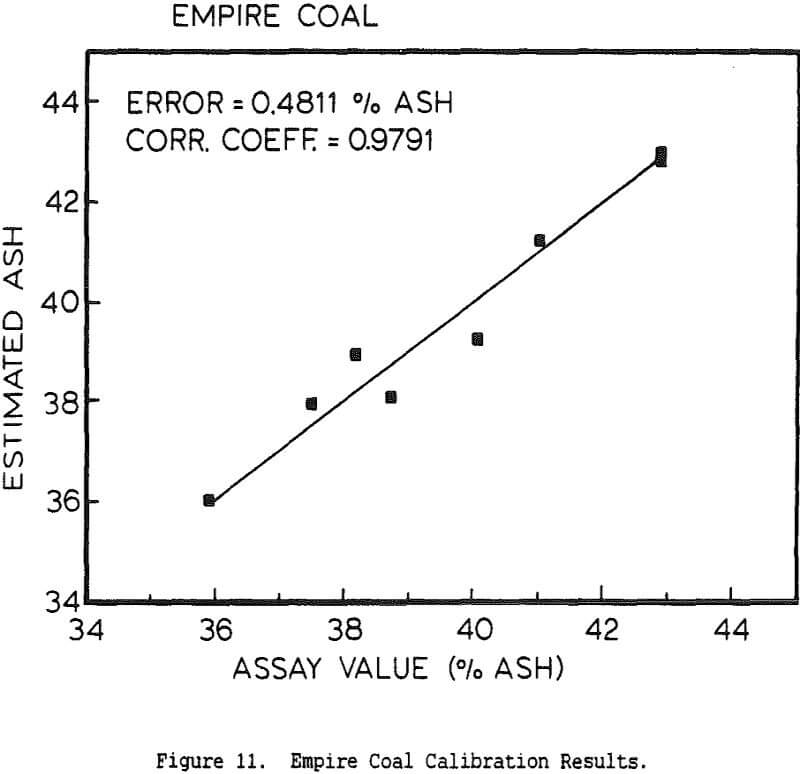
The use of a single detector for all of the measurements allowed the use of an electronics set which Outokumpu already manufactures, reducing the development and production costs. Using this unit several different tests were carried out. The first test used a mixture of synthetic ash and pure carbon, to determine how the instrument performed under ideal conditions. The second series of tests were intended to determine the performance for a range of coals, using coals collected from the USSR, Poland, and Great Britain. A final series of tests were then carried out using coals collected from an Ohio coal preparation plant, with the ash content ranging from 7 to 44% ash. The ash content in each case was calculated using empirical calibration equations of the form
%Ash = k0 + k1Fe + k2BS + k3DG
where Fe is the iron Kα fluorescence intensity, BS is the backscattered x-ray intensity, and DG is the y-ray transmission intensity. The empirical constants were calculated by linear regression.
The ash analyzer is a highly accurate instrument over a wide ash range. The estimate of the ash content is most accurate when the composition of the ash minerals is nearly constant, or changes slowly enough for the instrument to be recalibrated before the errors become too large. If the relative proportions of elements such as silicon and aluminum changes rapidly, this will cause changes in the effective atomic number of the low-atomic-number ash fraction, and there will be a loss of precision. Similar errors will result if the ratio for the various heavier elements to the iron content changes, or if the C/H/O ratio of the coal is altered. Because the composition of coal is highly complex, the only effective means for reducing these errors would be to measure the concentration of each element individually, which would greatly increase the cost while only giving a small benefit.