Table of Contents
Flotation test work on Bolivian tin ores some years ago led to the development of several workable schemes of selective soap flotation, but left unanswered many questions of soap-flotation chemistry. Accordingly, the study of the activation and soap flotation of quartz just reported was undertaken as a start toward the elucidation of some of the unanswered questions. The experimental work on quartz flotation consisted primarily of small-scale, vacuum-flotation tests in which activator and collector additions and pH were varied between wide limits.
Experimental Methods
The flotation technique and most of the reagent preparations were described fully in the previous paper. In addition, for the present work it was necessary to prepare satisfactory stocks of clean cassiterite and fluorite and to obtain samples of alizarin dyes.
Cassiterite: High-grade cassiterite from the Netherlands East Indies was crushed, sized, and cleaned carefully by gravity and magnetic separation.
Fluorite: Fluorite crystals free from interference colors were hand-picked from a stock of high-grade material (99.6 pct CaF2) originally obtained from Ward’s Natural Science Establishment.
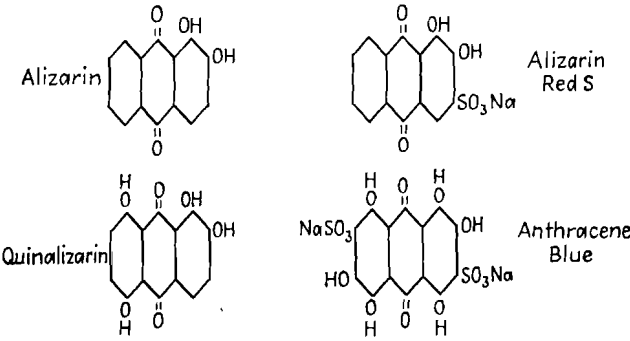
Alizarin Dyes: The four dyes tested as flotation reagents were the following:
Synthesis of Cassiterite and X Ray Studies
A variety of syntheses of crystalline stannic oxide are described in the literature, but experimental details and proofs of identity of the products are meager. Two methods were selected and tried:
- Reaction of SnCl4 and H2O in a hot tube.
- Crystallization from borax, stannic oxide melts.
The cassiterite used for flotation tests was prepared eventually by crystallization from borax melts, but some interesting results were obtained in attempting to react stannic chloride and water.
Reaction of SnCl4 and H2O: First, thermodynamic calculations were made which checked the feasibility of forming SnO2 at temperatures around 1000°C by the reaction:
SnCl4(g) + 2H2O(g) → SnO2 + 4HCl(g)
The thermodynamic data were taken from Kelley. After considerable preliminary experimentation the apparatus was set up as follows: The reaction tube was either an alundum or a Vycor combustion tube passing through a Burrell furnace which could be held at any desired temperature up to well over 1000°C. Stannic chloride gas and steam, each mixed with nitrogen, were introduced separately into the reaction tube. Quantities of SnCl4 and H2O were each controlled as follows: A metered flow of N2 was saturated with the vapor at a preset temperature by bubbling the N2 through the liquid (SnCl4 or H2O) held in a container in a heated oil bath. Vapor-pressure data from Stull’s compilation were used in calculating the flow rates of SnCl4 and H2O. Generally a small stoichiometric excess of SnCl4 was used.

Cassiterite Flotation
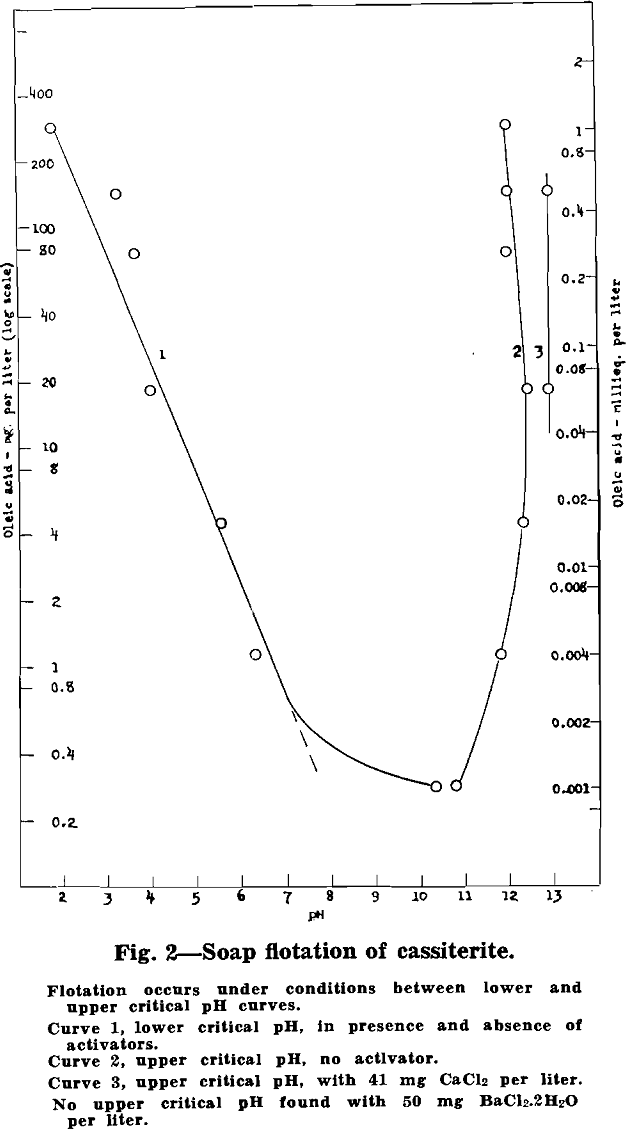
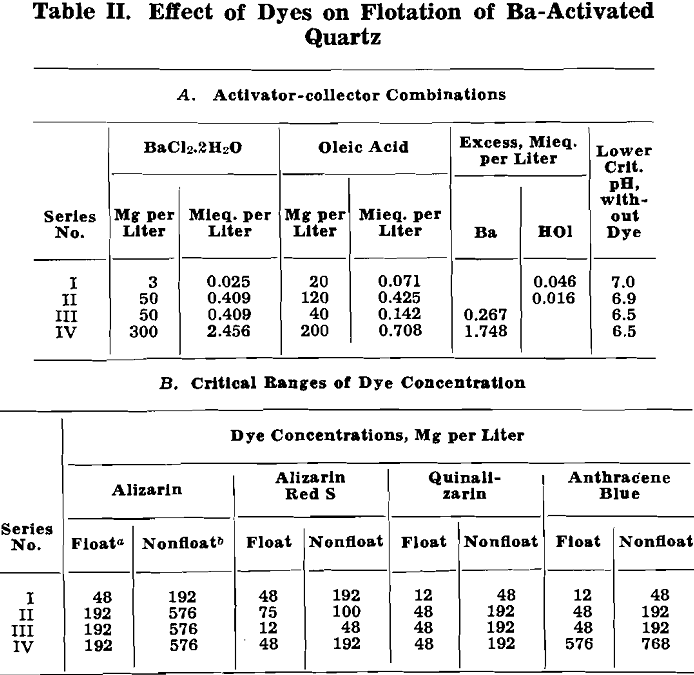
Vacuum-flotation tests were made with about 0.8 g of 2 to 20 micron artificial cassiterite per 100 ml pulp in each test. Critical pH values were determined for oleic acid additions from 0.28 up to 290 mg per liter. Exploratory tests were also made with BaCl2·2H2O (50 mg per liter) and CaCl2 (41 mg per liter) added to the pulp. The data are plotted in fig. 2:
Cassiterite floats readily without activation over a wide pH range. Both a lower and an upper critical pH were found, with flotation occurring over the entire range in between. The lower critical pH decreases with increase in added oleic acid, from about pH 7 with 1 mg oleic acid per liter down to pH 2 with about 300 mg oleic acid per liter.
The flotation of cassiterite with oleic acid at pH values as low as 2 and 3 was unexpected in the light of previous experience in the flotation of Bolivian tin ores. Additional vacuum-flotation tests with cleaned natural cassiterite checked the results with the artificial cassiterite.
Effect of Alizarin Dyes: Vacuum-flotation tests were conducted with varying quantities of alizarin, alizarin red, and quinalizarin and the following activator-collector combinations:
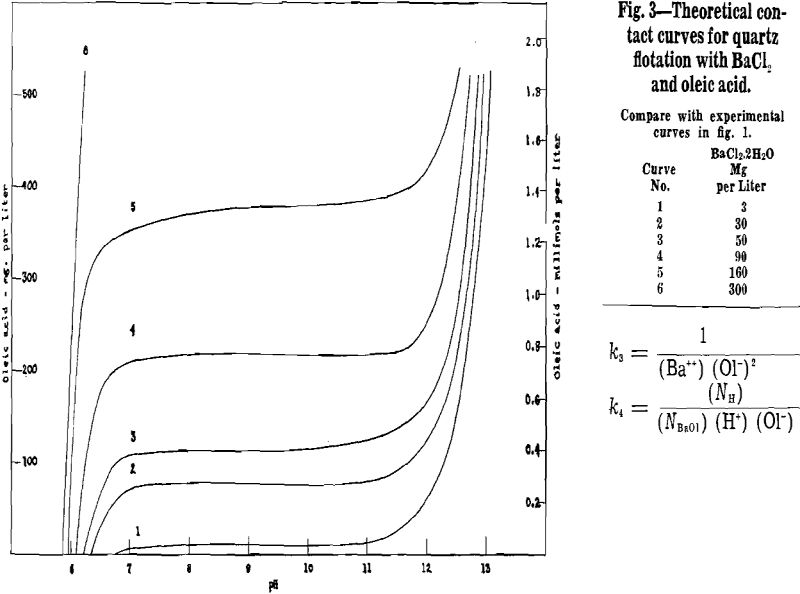
Lower and upper critical pH values for these tests are plotted in fig. 3, with pH values as abscissas and dye concentrations (log scale) as ordinates.
All the curves are of similar shape, showing that all three dyes act as depressing agents for cassiterite both in the absence of an activator and in the presence of Ba++ and Ca++. In general, as dye concentration is raised, the flotation range between lower and upper critical pH narrows until a dye concentration is reached above which flotation is not obtained at any pH.
In the absence of an activator, 200 or 300 mg per liter of any one of the three dyes was required to secure complete depression of cassiterite over the entire pH range. Such concentrations of dye correspond roughly in magnitude to a pound or so of dye per ton under ordinary flotation conditions. Moreover, these quantities of dye are two or three orders of magnitude greater than would be needed to film the cassiterite monomolecularly. Clearly, un-activated cassiterite is not depressed easily with small dye additions, and this may prove advantageous.
Fluorite Flotation
Like unactivated cassiterite, fluorite floats over a wide pH range and wide range of oleic acid concentrations. Also, the trend of decreasing lower critical pH with increasing collector concentration is repeated for fluorite. In the case of fluorite, however, the straight line drawn through the lower, critical-pH data has a slope of —1.0 and corresponds to the relation:
(critical H conc.)/(HOl addition) = 13
when (H+) and (HOl) quantities are expressed as mols per liter.
Like Ca-activated quartz and cassiterite, fluorite shows an upper critical pH. However, at high-collector concentrations the upper pH limit decreases so that with several hundred milligrams oleic acid per liter the fluorite does not float in alkaline pulps.
Reviewing data for both cassiterite and fluorite,
there are strong indications of specific depressing actions for the combinations calcium-quinalizarin, barium-quinalizarin, and barium-alizarin red S, and indications of specificity in alkaline pulps for the combination calcium-alizarin. On the other hand Ca did not act as a depression-sensitizer with alizarin red S, and Ba did not cooperate with alizarin.

Effect of BaCl2 and Other Activators on Soap Flotation of Quartz
Flotation of quartz is of practical importance as something to be avoided in soap-floating many types of ores. Clean, unactivated quartz is not floated with fatty acids and soaps, such as oleic acid and sodium oleate, in the quantities normally used for flotation. However, data in the literature indicate that almost any multivalent cation will activate quartz if given an opportunity. Thus, a common problem is to prevent activation of quartz by the various inorganic cations inevitably present in flotation pulps.
Preparation of Materials
Quartz: Large lumps of high-grade vein quartz were crushed dry in a cone crusher and rolls. The —20, +28-mesh portion was screened out and used in the subsequent steps. This material was passed through a high-intensity magnetic separator to discard iron, then leached twice with hot concentrated HCl and washed repeatedly with distilled water. The cleaned sand was then wet ground with porcelain balls in a porcelain pebble mill, deslimed repeatedly by settling and decantation to discard —800-mesh material, and again washed with hot HCl followed by distilled water. The resulting stock of quartz was stored under water. Chemical analysis gave 99.8 pct SiO2.
Oleic Acid: The preparation of oleic acid was based on fractional vacuum distillation of methyl oleate followed by regeneration of oleic acid, and finally fractional crystallization of oleic acid from acetone solutions at low temperatures. The pure oleic acid was stored in a refrigerator. The iodine number of the oleic acid was found to be 90.0 (theoretical 89.93).
Experimental Procedures
Vacuum Flotation: The flotation cell consists of a standard, glass-stoppered graduated cylinder of 100-ml capacity. A large number of these graduates are kept on hand so that a dozen or so tests can be in progress at the same time. For lecture demonstration purposes a 1-liter graduate has been used with substantially the same technique as that used with the 100-ml cylinders.
About 1.2 g (±10 pct) of quartz is scooped out of the stock (under water) and transferred to the cylinder with clean, air-saturated distilled water. Reagents are added and the volume brought to about 100 ml. The glass stopper is inserted and the pulp then agitated for 30 min by tumbling end over end at 50 rpm. For this purpose a pair of brackets holding up to 12 100-ml cylinders was built onto the slow-speed shaft of a 1/20 hp gearmotor.
Determination of Critical pH: The principal variables in this work were pH, activator concentration, and collector concentration. The usual procedure was to make up a pulp (as already described) in the 100-ml graduate with given quantities of activator and collector and with 2-mg terpineol per liter to act as frother. With this pulp a series of flotation tests was carried out at varying pH. Sodium hydroxide (carbonate-free) and hydrochloric acid were used for pH control, and a full conditioning period was provided after each pH change to insure equilibrium. The pH was measured with a Beckman Model H, line-operated pH meter.
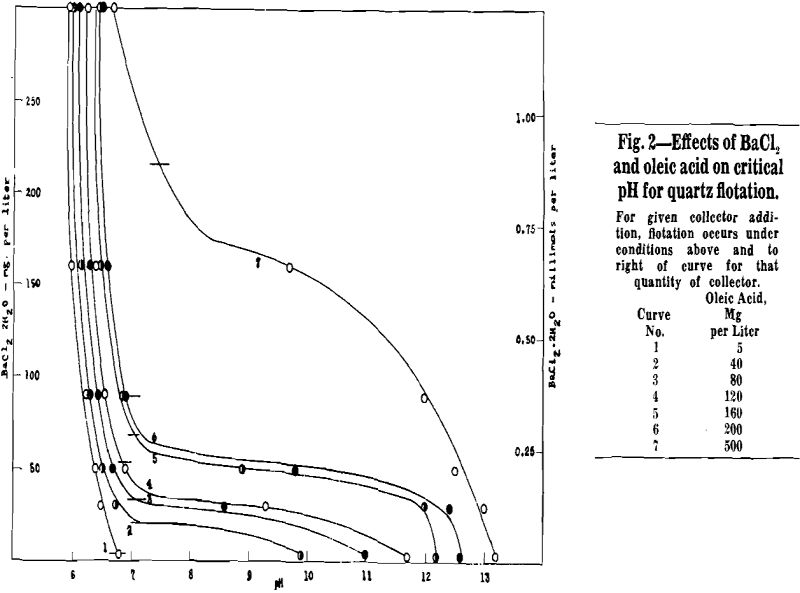
When barium is used as activator, no flotation occurs with a given activator-collector combination below a certain critical pH. As the pH is raised above this critical value, quartz floatability increases rapidly so that complete and rapid flotation is obtained at pH values from 0.5 to 1.5 units above the lowest pH at which slight flotation can be detected. The quartz remains floatable as the pH is increased further. With activators other than barium, the variation of quartz floatability with pH may be more complex. Not only is there a “lower critical pH” below which no flotation occurs, but also there may be an “upper critical pH” above which there is no flotation. A few cases of more complex behavior have been observed, which involve two pH ranges in which flotation occurs.
Barium Activation of Quartz
Experimental: Critical pH determinations were made over the following ranges of reagent additions:

These concentrations cover the usual range of practical reagent concentrations. Thus 5 mg per liter = 5 ppm = 0.01 lb per ton of water, which is equivalent to 0.04 lb per ton of ore in a pulp 20 pct solids. For the very dilute pulps (about 1 pct solids) used in this work, the quantities of reagents abstracted by the mineral can account only for a very small fraction of the total reagent addition, since a reagent addition of only 1 or 2 mg per liter of pulp would be sufficient to form a monomolecular film on the quartz.
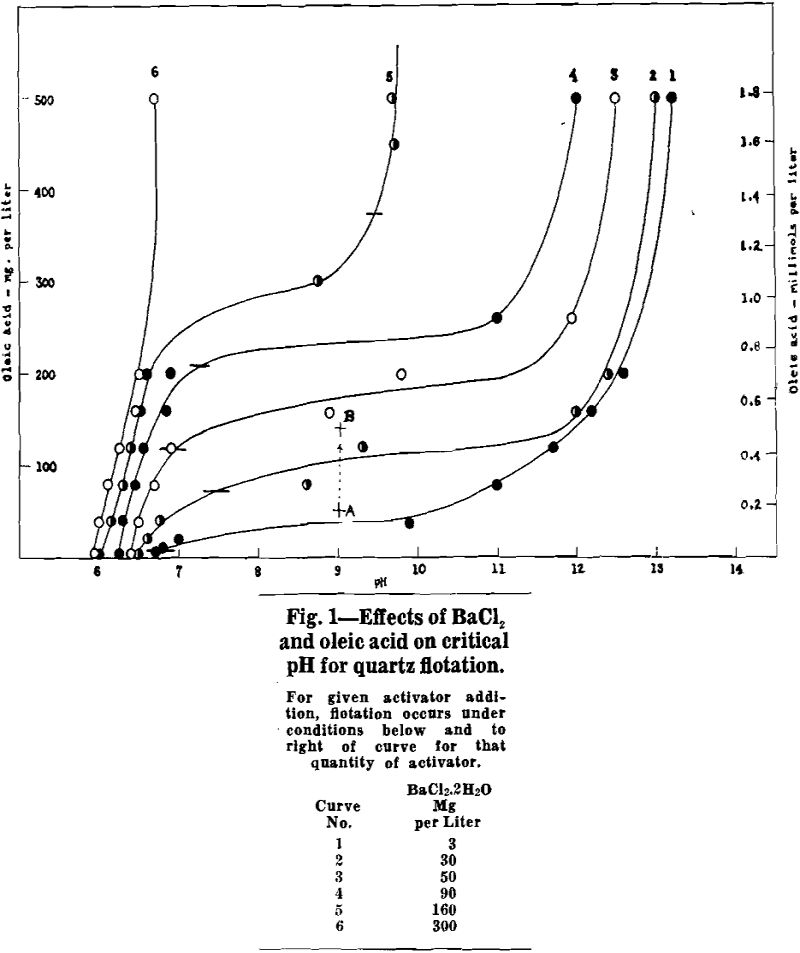
When barium is in excess of that required to form barium oleate, the critical pH falls in the range 6 to 7. No flotation occurs below the critical pH. Flotation occurs at all pH values above critical, including the pH of a normal sodium hydroxide solution. For the stoichiometric condition of excess barium, critical pH is relatively insensitive to reagent quantities.
When oleate is definitely in excess of that required to form barium oleate, the critical pH is well into the alkaline range so that flotation is possible only at a high pH. The critical pH is lowered and the pH range of flotation becomes greater as the quantity of activator is increased. For example, with 3 mg BaCl2·2H2O per liter and an excess of oleic acid the quartz floats only in strongly alkaline pulps above pH 13, but with 160 mg BaCl2·2H2O perliter and an excess of oleic acid the quartz floats at pH values as low as 11.

Activation, Collection, and Precipitation Reactions: Our data are consistent with the view that the quartz flotation is activator-controlled and not collector-controlled, provided only that there is enough oleate in the system to film the quartz when it does become activated. Two phenomena which would be expected in a collector-controlled system are absent: (a) There is no upper critical pH, corresponding to displacement of Ol- by OH- and depression as the pH is raised; (b) Even with a large excess of barium, several times more than required to precipitate the oleate as insoluble soap, no difficulty was experienced in collecting quartz, in fact, flotation was best under these circumstances.
The following equation may be written to represent the activation reaction at one locus of ruptured bond on the quartz surface:
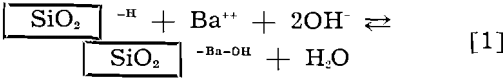
![]()
The formation of a barium-soap precipitate (independent of the presence or absence of quartz) is represented by:
Ba++ + 2 Ol- ↔ BaOl2 (ppt)…………………………………………………………………[3]
The depressing effects of lowering pH and increasing Ol- are accounted for by the following reaction:
![]()
This equation shows floatable quartz, filmed with oriented, partial monomolecular BaOl+, returned to its original nonfloatable condition by replacing the BaOl+ with H+ and forming a separate BaOl2 precipitate which does not help the quartz to float. Since both H+ and Ol- are reactants on the left-hand side of the equation, it is clear that decreasing pH and increasing Ol- favor the formation of an insoluble barium oleate in preference to a BaOl film on the quartz, in agreement with our experimental results. A semiquantitative explanation of the experimental results, based largely on Eq 4, is developed in the subsequent sections.
Adsorption Equilibria and the Equilibrium Constant: For any chemical change at constant temperature, represented by
aA + bB + cC ↔ dD + eE + fF………………………………………………………….[5]
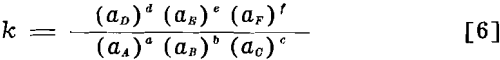
in which aA, aB, etc., represent the activities of the respective reactants relative to some arbitrarily fixed standard state for each reactant. This is a general relationship based on the second law of thermodynamics which applies whether the reactants are pure solids, liquids, or gases; or in liquid or solid solutions; or in surface films, physically or chemically adsorbed; or in colloidal dispersions.
Application of Mass Action to Reactions in Quartz Flotation: For reactions 1 to 4 in the quartz-flotation system, the mass-action constants can be written as follows, assuming ideal Langmuirian behavior of adsorbates:
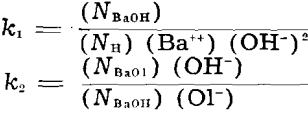
The equilibrium constant k, deals with the adsorption of Ba++ on quartz in the absence of oleate, for which we have no data. Since, as already discussed, reaction 2 goes strongly to the right under all conditions tested we can say only that k2 is very large and that no appreciable quantity of adsorbed BaOH is present in the systems with oleate added. In other words, the elementary spaces available for ion adsorption are occupied either by H or by BaOl or they are empty.
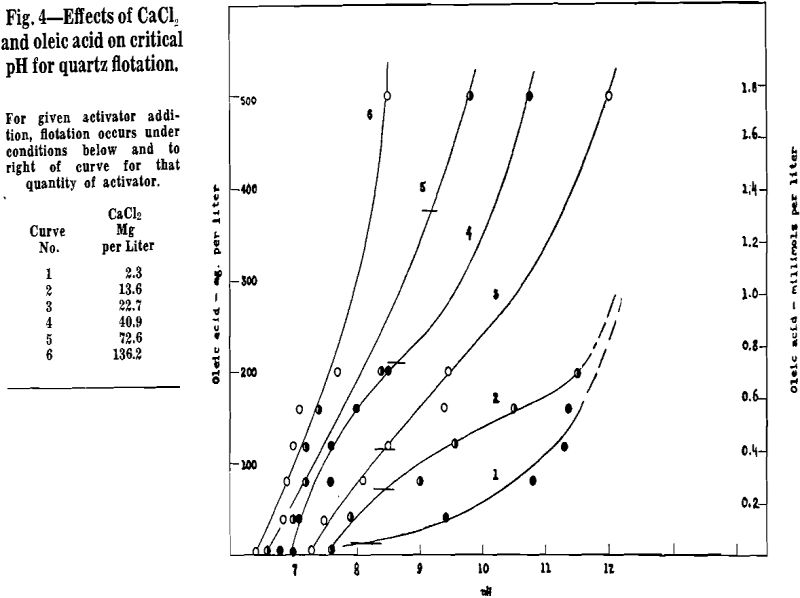
Calcium Activation of Quartz
Vacuum-flotation tests of calcium activation were made over the following ranges of reagent additions:

Calcium, unlike barium, exhibited an upper critical pH above which the quartz did not float. Because of difficulties in measuring the very high pH values, no pH data are reported. However, quartz did not float with Ca activation in 1-normal sodium hydroxide. These observations correlate with the fact that Ca(OH)2 is less soluble than Ba(OH)2. Hence it is possible that nonflotation above the upper critical pH is due to displacement of Ol by OH at the Ca-activated surface; that is, the Ca reaction corresponding to Eq 2 is reversible at high pH values. Another explanation is that above the upper critical pH the Ca precipitates as Ca(OH)2 leaving the Ca++ concentration in solution too low to maintain activation.
Aluminum Activation
Before making flotation tests with aluminum as activator, a series of potentiometric titrations were made to gain a little acquaintance with the chemical reactions in solutions to which aluminum and
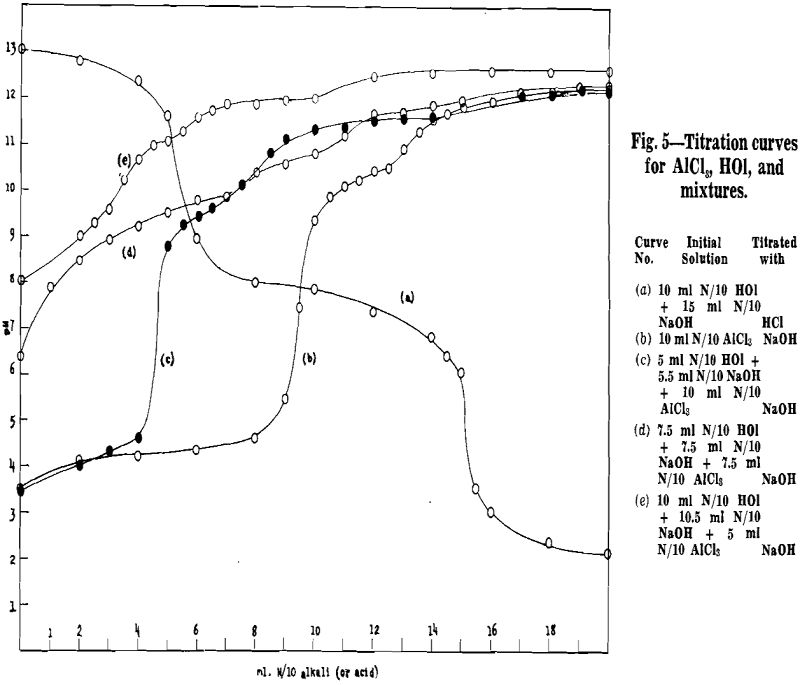
oleic acid are added in varying amounts.
The vacuum-flotation tests of aluminum activation covered wide ranges of reagent additions in an exploratory fashion:

As might be expected, flotation behavior with Al-activation is somewhat more complex than with Ba-activation. In particular, Al-activation showed both a lower and an upper critical pH, with flotation between the two, and nonflotation outside the two limits.
The upper critical pH falls in the range 12 to 13, regardless of the quantities of activator and collector added. This upper pH limit to quartz flotation with Al-activation may be accounted for by the fact that Al+++ is converted to AlO2- at high alkalinities.

