The practical application of common alkaline inorganic chemicals such as lime, soda ash, caustic, silicates and phosphates, in the beneficiation of minerals, is well known within the industry. These materials have been found to be useful throughout the flotation circuit. This paper discusses the chemistry of the soluble sodium silicates in relation to their use in flotation regulation as depressants, activators and dispersants.
The chemistry of the soluble silicates in the pure solution state has been a subject of great interest for many years, but has only recently, through the use of nuclear magnetic resonance (NMR) spectroscopy, been shown, to be more complex than earlier imagined. Due to this complex nature, combined with an apparent facile exchange between the various possible polymeric species present, one is forced to study the chemistry from a systems oriented perspective. In addition, the complexity of the chemistry of mineral systems rivals that of soluble silicates; thus when the two systems are combined one is generally faced with a major challenge to fit theory with practice. Taking into consideration the challenge inherent in studying these systems, this report has been developed to familiarize you with the current state of knowledge in silicate chemistry and the implications of this knowledge for current and future studies of, and uses for, silicates in mineral beneficiation.
Chemistry of Silicates
The soluble sodium silicates have the general formula:
Na2O·mSiO2·nH2O
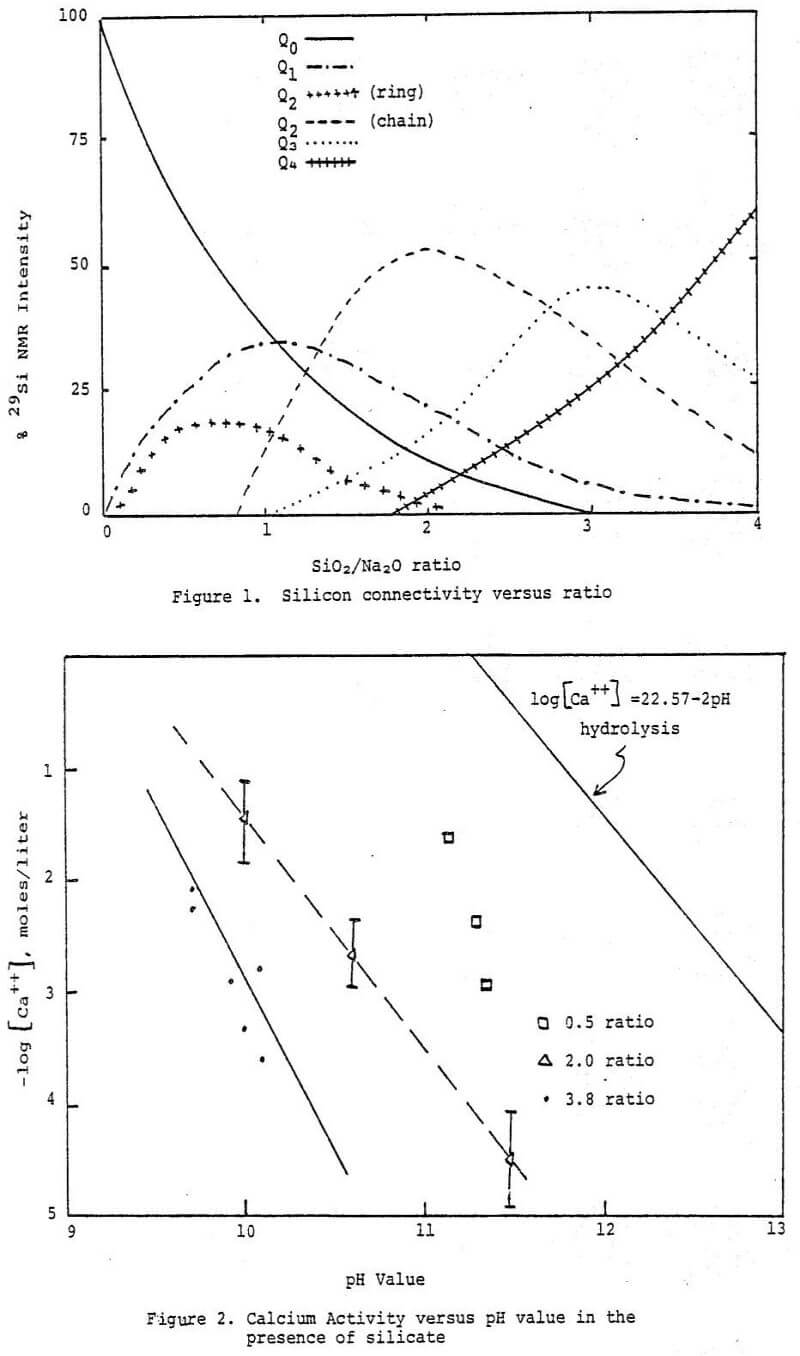
and are made as a glass from a very pure form of quartz sand and natural sodium carbonate in an open hearth regenerative furnace. The molecular ratio of silicon (as SiO2) to sodium (as Na2O) is often called the silicate ratio or modulus. Knowledge of this characteristic of a sodium silicate is very important in understanding the chemistry of these materials. Molten compositions of Na2O·mSiO2 of any proportion can be prepared; however only those with values of m between -1.6 and 3.75 are usually available commercially in solution form. Manufacturers sell either concentrated aqueous solutions, glass lumps which can be dissolved on site in suitable pressure dissolvers, or hydrous powders which can be readily dissolved at atmospheric pressure.
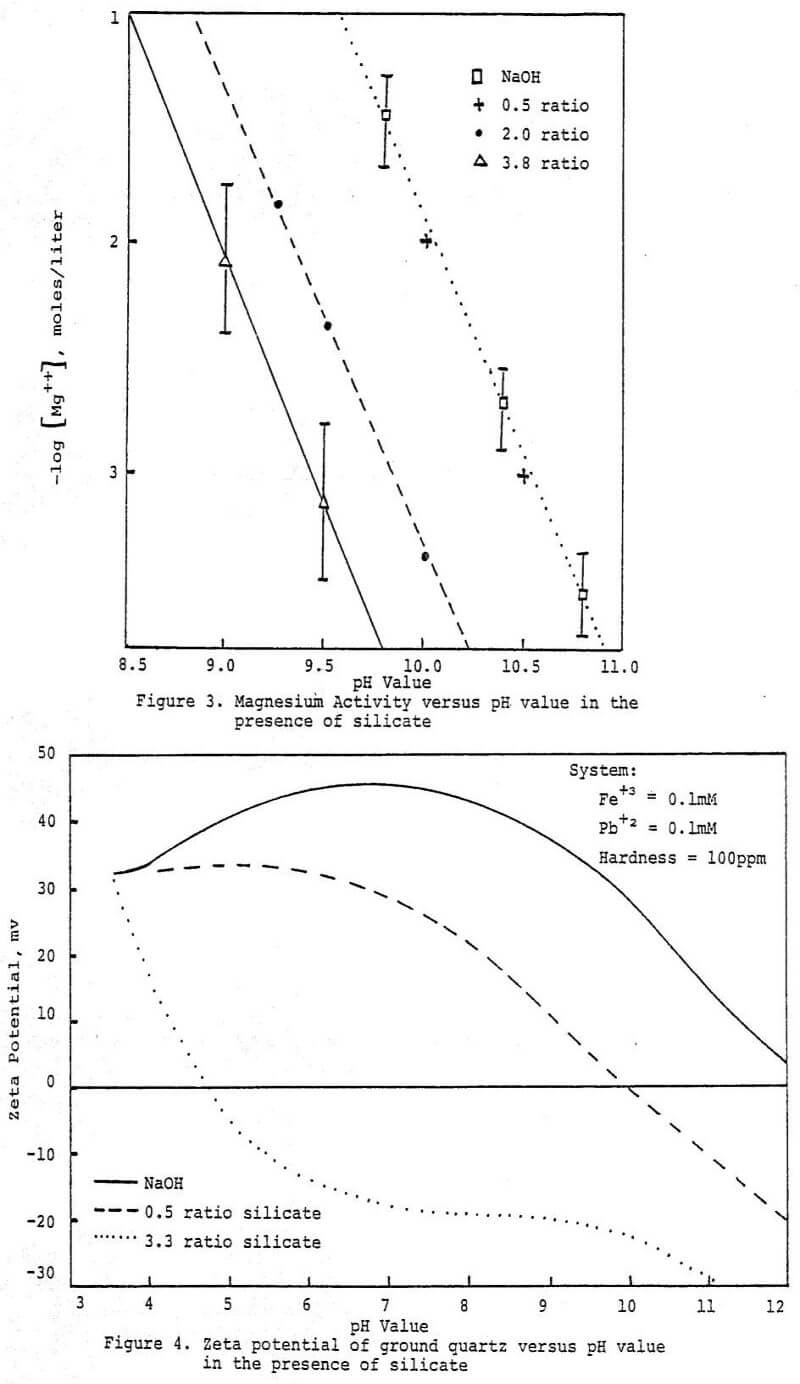
In summary the above studies suggest that silicate, maintains surfactant activity via a dual mechanism; through reaction with divalent metal cations and through reduction of specific surfactant adsorption onto the solid surfaces present.
It would appear that there is a strong tendency for soluble silicate species to preferentially adsorb onto activated sites on oxide surfaces. This would explain the reduction in surfactant retention by mineral surfaces and help maintain substrate water wettability. The tendency of silicate anions to condense through the silanol groups possibly creates a situation favorable to the formation of an irreversibly adsorbed layer of silicate. Further studies are underway to explore in greater depth this concept and its implications.
The above studies point out how the complex chemistry of the soluble silicates govern their behavior at mineral surfaces. Their dispersant properties follow from the ability of the more polymeric forms to maintain a net negative charge on oxide surfaces. It appears that the mechanism is related to the association of multivalent metal ions with the active surface silanol groups on the colloidal silicate anions either in solution or at the suspended particle interface. Association of polymeric soluble silica at the suspended particle interface might be enhanced by mutual interaction between adsorbed silicate species, leading to a resurfacing of the particle. This resurfaced particle would be expected to be quite hydrophilic, thus depressed in a typical froth flotation system. Increased water wetness would be due to both the natural hydrophilicity of condensed polymeric silicate anions and the inhibition of hydrophobic collector adsorption. Excess use of soluble silicates could have the effect of depressing all particles because of its affinity for most oxide surfaces. Also, the corrosion inhibiting properties of silicates are most likely due to their affinity for the active sites that arise at the onset of corrosion. The silicate species apparently complex with the active sites and sterically block further reaction.
![]()