Table of Contents
Spectrochemical methods were developed to determine beryllium in the concentration range 0.0015 to 4.0 percent in siliceous mineral beneficiation products. Two methods are described. They are (1) a fusion-pellet-spark procedure (spark method) and (2) a sustaining alternating-current arc procedure (arc method). In the spark method the sample is fused with lithium tetraborate and vanadium pentoxide. The resulting bead is crushed, mixed with powdered graphite, and briquetted. This is analyzed spectro-graphically using controlled high-voltage spark excitation. In the arc method the sample is diluted with germanium metal, graphite, and vanadium pentoxide. Weighed charges are packed into cupped graphite electrodes and subjected to excitation with a sustaining alternating-current arc. Vanadium serves as internal standard for both methods. Effects due to matrix variations in samples and standards are eliminated either by fusion with lithium tetraborate in the spark method or by dilution with germanium and graphite in the arc method. Beryllium determinations show deviations from accepted chemical values of approximately 5 and 8 percent for the spark and arc methods, respectively. Analyses can be made more rapidly by the arc procedure with a sacrifice in precision and accuracy. Either method can be applied to the determination of beryllium in silicate rocks.
Beryllium Alloys
Beryllium alloys and compounds are required for applications in the fields of aircraft, atomic energy, and space vehicles. Future needs will require sources of this element that are not presently being mined.
Bureau of Mines research activities at the College Park Metallurgy Research Center, Maryland, have included efforts to economically recover beryl from low-grade ores.
In connection with the work reported by Shelton, the need existed for a rapid, accurate method to determine the beryllium content of siliceous products resulting from beneficiation tests. Petrographic examination of beneficiation products showed the minerals present in major amounts to be feldspar, quartz, and muscovite. Lesser quantities of beryl, garnet, pyrite, and iron- magnesium minerals were also present. Wide variations occurred in the proportions of the various components in the beneficiation products. Consequently, any method of analysis for the determination of beryllium in these products must be insensitive to major compositional differences among samples. The concentration range to be expected was from 0.002 to 4.00 percent beryllium.
Chemical determinations for beryllium in concentrations below 0.01 percent usually require separation procedures that complicate the analysis and thus affect the accuracy of results. Two publications describe sensitive fluorometric methods.
The advantages of optical emission spectrochemical methods of analysis include speed, high sensitivity, and usually increased accuracy and precision over chemical methods in the low concentration ranges.
A number of publications describe specific spectrochemical procedures for the determination of beryllium content in rocks, ores, and mineral products. Marks and Jones have presented a total energy method. Creitz has described a fusion-pellet-spark procedure. Others employing arc-type excitation have been reported by Pieruccini and by Alekseeva and Rusanov.
Several techniques have been used by the Bureau at the College Park Metallurgy Research Center. These include direct-current arc procedures employing the total energy principle both with and without the use of internal standards. Several diluents were tried with these methods, including lithium carbonate, copper oxide, and germanium metal. A solution-spark method was also used. In this procedure the sample was fused and dissolved in acid, and the solution was sparked using the porous cup arrangement as described for the analysis of titanium. These methods, although useful for the analyses of many sample types, did not produce results of sufficient accuracy to permit calculation of metallurgical balances from the beneficiation tests.
Two approaches to this problem were undertaken, and the investigations were carried on concurrently. These resulted in the development of two methods, one employing a fusion-pellet-spark technique, and the other a powder technique utilizing the sustaining alternating-current arc for excitation.
Outline Beryllium Assay Methods
Fusion-Pellet-Spark Method
Spectrochemical procedures employing spark excitation of samples invariably yield more precise results than those employing direct-current arc excitation. This is due primarily to the close control maintained over the electrical parameters of the spark circuit that affect the character and reproducibility of the spectra. Also, greater precision can be expected from random sampling of the specimen because of the intermittency of the spark.
The procedure as developed is similar in basic principles to those described by Price, Tingle and Matocha and Creitz however, the details differ considerably.
Part of the pulverized sample is fused with lithium tetraborate (Li2B4O7,) and vanadium pentoxide (V2O5). The resulting glass bead is crushed, mixed with powdered graphite, and briquetted. This is analyzed spectrographically using controlled, high-voltage spark excitation. The sample pellet is rotated during the excitation period. Spectra are recorded photographically and evaluated by the usual methods of photometry. Vanadium serves as the internal standard for the determination of beryllium over the concentration range from 0.0015 to 4.0 percent.
Sustaining Alternating-Current Arc Method
Rozsa and Uguccini have described the sustaining alternating-current arc and claim certain advantages for it over the direct current arc as a spectrographic source. Chief among these are increased precision and sensitivity of detection for the refractory elements.
A common practice in the College Park laboratory is to use a mixture of germanium metal and graphite as a diluent in procedures for the analysis of many types of powdered samples. Excitation, however, is by means of the direct-current arc. The advantages of using germanium as a diluent are described by Strock and include (1) more nearly parallel volatilization behavior for many elements, (2) a liquid temperature range (958° to 2,700° C) conducive to the formation of homogeneous melts with many sample types, (3) a favorable ionization potential (8.13 volts) with respect to elements that may be determined, and (4) the ability to contribute conducting particles to the arc.
The method as developed represents an attempt to take advantage of the desirable characteristics of germanium as a diluent and the reproducibility of the sustaining alternating-current arc source.
Part of the sample is diluted with a mixture of germanium metal, graphite, and vanadium pentoxide. Weighed charges of this mixture are packed into cupped graphite electrodes and analyzed spectrographically using the sustaining alternating-current arc. Vanadium is used as internal standard for the determination of beryllium over the concentration range from 0.003 to 2.00 percent.
Details of the two procedures, including standardization, operating conditions, and results of analyses, are given in subsequent sections of this report.
Equipment
Excitation for the two methods is obtained from a commercial spectrographic source unit capable of providing a high voltage, air-interrupter-type spark and a sustaining alternating-current arc. The specific excitation parameters for each method are listed in sections describing the procedures.
The spectrograph used in Dual Grating type providing an average reciprocal linear dispersion of 4 angstroms per millimeter in the first order from each grating. For the two procedures described in this report, however, one grating only is used. External optics consist of a step-filter lens at the slit, cylindrical lens, biprism, primary aperture, and two spherical lenses. The spherical lenses produce an image of the discharge at the primary aperture This image is focused onto the gratings. Other items of equipment and accessories are listed in table 1.

Standards
Four pulverized beryl-containing ore samples were chemically analyzed in several laboratories by independent methods. Values of 4.04, 1.99, 0.50, and 0.094 percent Be are considered to be correct, and these samples are used as primary standards for each method. Other standards were prepared to obtain intermediate values by blending two of these. To extend the range to lower concentrations, dilutions were made in beryllium-free anorthoclase, (Na, K)2 O-Al2O3, · 6SiO2. A separate series was prepared by adding beryllium oxide to quartz and making successive dilutions. Dilutions were mixed in plastic capsules by means of a mixer-mill. Samples and diluents were ground to minus 200-mesh prior to blending. Standard pellets for the spark method were prepared by combining portions of the 0.094 percent standard and anorthoclase into fusion mixtures. Details are given in the section describing the procedure for this method. The compositions of the standards are listed in table 2.

Experimental
Vanadium was selected for use as possible internal standard to be added to samples and standards in constant amount. A number of vanadium spectral lines of suitable intensity occur in the same wavelength region as the beryllium lines to be used for analysis. Excitation potentials for the vanadium- and beryllium-ion lines employed are somewhat similar, although not ideally matched-approximately 13 electron volts for beryllium and 11 electron volts for vanadium.
Moving plates were used to study the volatilization behavior of beryllium and vanadium. Data obtained were plotted as percent transmittance versus time in seconds. Figure 1 shows typical volatilization patterns of the two
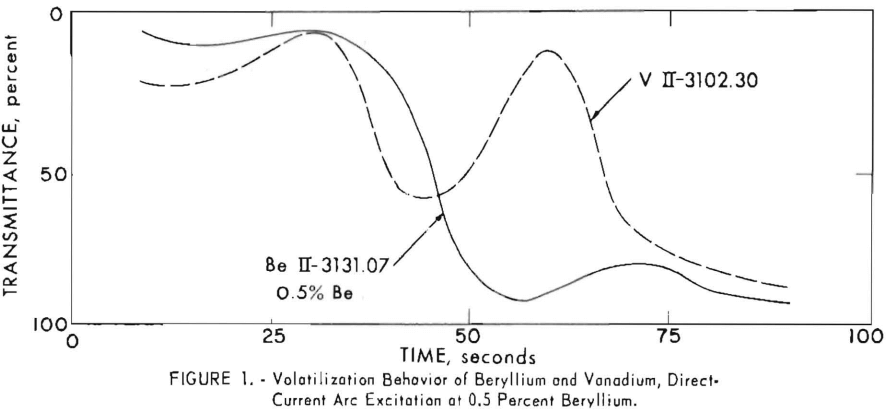


elements when a 15-ampere direct-current arc was used as the excitation source. Figures 2, 3, and 4 show patterns for the two elements at various concentrations of beryllium when the sustaining alternating-current arc at 20 amperes was used. Data for these curves were obtained from samples mixed with a diluent containing germanium metal and graphite. Proportions used were 1 part sample, 9 parts germanium, and 10 parts graphite. Vanadium in solution was added to the diluent (0.025 percent) as internal standard and was present in constant amount in all sample charges. Weighed charges of 15 milligrams were used. Details for the preparation of the diluent and sample charges are given in the section, “Procedures.”



The direct- and alternating-current arcs produced similar volatilization patterns for the two elements. The vanadium shows a pattern with intensity maxima and minima that are more pronounced for the higher beryllium concentrations. At a concentration of 0.5 percent, beryllium was volatilized in less than 50 seconds with the alternating-current source. The beryllium persisted for at least 90 seconds with the direct-current arc. Beryllium-vanadium intensity ratios obtained with the direct-current arc were not adequately reproducible; therefore, the direct-current source was abandoned for this analysis. The volatilization curves show that it is necessary to arc for 90 seconds with the alternating-current arc to completely volatilize the vanadium; consequently, a period of 90 seconds was selected. Beryllium-vanadium intensity ratios of adequate reproducibility were obtained, and analytical curves were established to cover the concentration range from 0.003 to 2.0 percent.
The volatilization behavior of beryllium and vanadium using the fusion-pellet spark procedure is illustrated in figure 5. The nearly parallel behavior undoubtedly accounts in large measure for the greater precision obtained for this method compared with the alternating-current arc procedure. Exposure times of 12 to 48 seconds provided beryllium-vanadium intensity ratios suitable for the preparation of analytical curves in concentration ranges from 0.0015 to 4.0 percent.
Matrix variations in samples and standards caused no difficulty in either procedure. For the alternating-current arc method blends of the primary standards were used to obtain intermediate points on the curves shown in figure 6. Standards below 0.094 percent beryllium were prepared by diluting in anorthoclase. Values plotted on figure 7 are alined with higher values obtained from chemical analysis of naturally occurring samples. Figure 8 shows analytical curves for the spark method prepared from chemically analyzed natural samples. Values obtained from standards in a quartz base are in agreement with those

obtained from chemically analyzed natural samples diluted in anorthoclase. On figure 9, values at 0.06,
0.03, 0.01, and 0.005 percent beryllium are from standards in a quartz base. Further evidence that matrix differences in samples analyzed caused no difficulty is provided by the agreement between chemical and spectrochemical values as shown in table 3. The chemical method employed was a fluorimetric procedure modified from that described by Fletcher, White, and Sheftel.
Borates in Glass
The advantages of using boron in glass were established in the latter part of the 19th century by work on technical and optical products. A great deal of the development and production was in Germany, and elsewhere in Europe, during the early 1900’s. World War I disrupted supplies of technical glass so development of suitable compositions began in the United States. Borosilicate glass for laboratory, optical, heat resistant, and other technical use was soon available worldwide, and as new technology such as x-ray diagnosis came along, special compositions were worked out and marketed. Because boron provided lower- expansion glasses, good resistance to thermal shock, and improved durability, compositions for laboratory glassware, industrial piping, gage glass for boilers, household ovenware, high-voltage electronic tubes, and many other uses were marketed. Morey (1938) gives data for many early borosilicate glasses.
Following World War II, various electronic technologies developed rapidly, bringing further demands for borosilicate glasses. Pharmaceutical containers were required in large quantities, and very-good-durability glasses were soon being produced in larger continuous furnaces. The development of fiberglass in the late 1930’s was based on borosilicate glass for textile products, using a low-alkali composition marketed initially for electrical uses and called E glass (7 in Table 1). In the late 1940’s other boron-containing glasses were developed when it was found that the standard soda-lime compositions did not provide resilient, durable fiberglass insulation (Gagin, 1984). Of all the new uses for boron in glass, fiber glass for textiles and insulation proved to be the largest in volume. Table 1 gives some typical glasses developed with boron as a primary component to suit specific end uses.
1. older chemical resistant glass 2. Corning 7740 heat and chemical resistant glass 3.,4. electronic glass 5. optical lenses 6. solder glass for sealing electrical components 7. E glass 8. soft glass for making insulation by rotary fiberization
The two basic boron products used in the 1940’s for glass were boric acid and borax. They were refined products, produced primarily in California, but also at other locations with borate ores. As the demand for boron products increased, and plants to produce fiberglass were built near population centers, the high cost of shipping boric acid and 10-mol borax from California pushed development of more efficient raw materials specifically for the fast-growing fiberglass industry. Anhydrous borax became a widely used material for insulation, and demand strained producer capacity because production equipment to remove the water from borax was expensive. Producers and consumers jointly developed suitable products meeting all the specifications of the textile and insulation industries. Anhydrous boric acid was marketed, with reduced shipping costs per unit of boron and improved melting characteristics, but high production (dehydration) costs limited its use to E glass for textiles. Rasorite products were also introduced, and were essentially impure borax of varying hydration. Two products were offered, one being anhydrous (Rasorite) which found major uses in the United States for a number of years owing to its 65% boron oxide content which lowered shipping costs compared to those of the 46% boron oxide product (Rasorite).
Borates In Glass by Gagin, Lawrence V.
Organization: The American Institute of Mining, Metallurgical, and Petroleum Engineers
Pages: 4, Publication Date: Jan 1, 1985
Procedures
Fusion-Pellet-Spark Method
Sample and Standards Preparation
Samples are prepared in this manner. The following materials in the quantities given are placed in a plastic capsule: 2.29 grams of lithium tetraborate (Li2B4O7), 250 milligrams of sample or standard, and 25 milligrams of vanadium pentoxide (V2O5). The capsule is capped tightly and shaken on the mixer-mill for 3 minutes without mixing balls. The contents of the capsule are carefully transferred to a ¾-inch-deep graphite crucible and placed in a muffle furnace at 1,000° C for 15 minutes. The crucible is removed from the furnace, and the fused melt is poured immediately into a shallow-cupped graphite crucible and allowed to cool. The cooled bead is placed in a plattner mortar and broken into small pieces. These pieces are transferred to a tungsten carbide capsule with two tungsten carbide grinding balls, placed on the mixer-mill, and ground for 3 minutes. Approximately three-fourths of the pulverized bead is screened through a 200-mesh sieve and put in a labeled glass vial; the remainder is discarded. Three hundred milligrams of the screened pulverized bead is mixed with 600 milligrams of pelletizing graphite in a plastic capsule. Mixing is

accomplished with the mixer-mill; however, no mixing or grinding balls are used. The contents of the capsule are transferred to the mold of a briquetting press. A ½-inch-diameter pellet, approximately 1/8-inch thick, is formed by applying pressure at 60,000 pounds per square inch for 15 seconds. The flat surfaces of each pellet are rubbed lightly on a piece of smooth paper to prepare the surface for sparking.
The pellets are somewhat hygroscopic and are therefore stored in a desiccator.


Standard pellets are prepared in the manner as described for the samples. However, for standards below
0.094 percent beryllium, dilutions of standard 36 are made during the fusion step. The appropriate amounts of standard and anorthoclase diluent are mixed with the lithium tetraborate and vanadium
pentoxide prior to fusion.
The standard beads so produced are then made into pellets. To prepare standard pellets with concentrations of beryllium below 0.00188 percent, dilutions of standard 36-G are made. Extremely small weighings are avoided by preparing a beryllium-free “blank” bead from anorthoclase, lithium
tetraborate, and vanadium pentoxide. This is ground to minus 200-mesh and then used as a diluent to make standards by successively diluting the pulverized bead of standard 36-G. These mixtures are made
into pellets with graphite, as described for the samples, and stored in a desiccator.
Excitation, Exposure, and Development Conditions
The lower electrode clamp of the arc-spark stand accommodates the pellet holder. A motor, contained within the stand, provides for rotation of the pellet holder at 10 revolutions per minute. The pellet is placed in the holder, a 3/16-inch-diameter graphite electrode (type 105-U) is held in the upper clamp, the gap is adjusted to 2 millimeters, and the spectra are produced using the excitation conditions given in table 4. The exposure and development conditions are given in table 5.


Photometry
The emulsion calibration curve is established using a direct-current iron arc and a 2-step filter, ASTM Designation E 116. Data from a preliminary curve are used to construct the final calibration curve. This is plotted in two segments according to the method described by Harvey.
The analytical line pairs and concentration ranges are listed in table 6. Typical analytical curves covering the various concentration ranges are presented in figures 6, 7, 8, and 9.
Transmittance measurements of the pairs of analytical and internal standard lines listed in table 6 appropriate to the concentration range are converted to intensity ratios by means of the emulsion calibration curve. For each concentration range, the intensity ratios obtained from spectra of standards are plotted relative to concentration on logarithmic coordinates. Analytical determinations are made by converting transmittance measurements for line pairs in the spectra of unknowns and control standards to intensity ratios, and the concentrations are read from the analytical curves. Average values obtained from duplicate spectra of the same pellet, corrected for any curve shift by at least two control standards on the same plate, are reported for each determination. No background corrections are necessary.
Sustaining Alternating-Current Arc Method
Sample & Standards Preparation
A diluent composed of 9 parts germanium metal, 10 parts graphite, and 0.025 percent added vanadium as internal standard is prepared as follows. High-purity, polycrystalline germanium metal is ground in plastic to minus 200-mesh. A standard solution of vanadium, 1 milligram per milliliter, is prepared by dissolving 178.5 milligrams of vanadium pentoxide in HNO3 and diluting to 100 milliliters. Thirty grams of graphite powder is weighed into a vycor dish and placed on a hot plate. Dropwise additions of the solution are made, totaling 14.25 milliliters. Care is taken to avoid contact of the solution with the dish. By maintaining the dish and contents hot, the solution evaporates readily from the graphite and contact of solution with the dish is avoided. This operation is performed in a well-ventilated hood. The mixture is heated over a meker burner for 10 minutes. After cooling it is transferred to a plastic capsule and 27 grams of the pulverized germanium metal is added. This is mixed on the mixer-mill with three plastic balls for 10 minutes.
Standard samples are prepared according to the procedure described in the section on “Standards.” The powder standards and samples are prepared for arcing by the following procedure. Twenty-five milligrams of pulverized sample (minus 200-mesh) is mixed with 475 milligrams of the germanium-graphite-vanadium diluent. Mixing is done in plastic capsules, using two 1/8-inch plastic balls, for 55 seconds. The mixer is a vibrating-type dental amalgamator. Fifteen milligrams of the diluted sample or standard is weighed into a 3/16-inch-diameter graphite electrode (type 105-S) , and the charge is tamped with the polished end of a glass rod.
Sample Excitation, Exposure, and Development Conditions
The sample-containing electrode is placed in the lower clamp of the arc-spark stand. The upper clamp is fitted with a 3/16-inch-diameter graphite (type 105-U) electrode. The analytical gap is adjusted to 4 millimeters, and the spectra are produced using the sustaining alternating-current arc source. Excitation, exposure, and development conditions are given in table 7.


Photometry
The emulsion calibration curve is established according to the procedures described for the fusion-pellet-spark method. Analytical curves are prepared from spectra of standard samples using the line pairs for the appropriate concentration ranges as listed in table 6. Curves are plotted as described for the spark method. Values for unknown samples are obtained from spectra of duplicate samples. Control standards are exposed on each plate to correct for any possible curve shift. Background corrections are not necessary.
Analytical Results
Typical results of spectrochemical analyses by the two methods described are shown in table 3, together with chemical values where available, of the same samples. The accuracy of the results on individual samples depends upon the validity of the primary calibration standards and the reliability of chemical analyses of comparison test samples. Repeat analyses of primary standards show deviations from accepted values of approximately 5 percent for the spark method and 8 percent for the arc method. Chemical results on individual test samples at concentrations above 0.05 percent beryllium show the same deviations from spectrochemical values.
Tables 8 and 9 give the methods of calculation. Results shown in table 8 are from analyses by the spark method; those in table 9 are from analyses by the arc method. Each beneficiation test provides a number of individual products with varying concentrations of beryllium. For each test, the analyses of the products are used to determine the percent beryllium recovered, and this value, the calculated feed, must agree with the analysis of the feed to produce a material balance. The calculated feeds for 10 tests are shown in table 10. All samples in each test were analyzed by the spark method; samples in four tests were analyzed also by the arc method and by chemical means. Errors in the recovery of individual products, in sampling and in weighing that originate outside the spectrochemical laboratory, are possible causes of deviations between analyses of feeds and calculated feeds.


Table 11 presents the results of a sampling experiment. Four split-out portions of feed sample were prepared outside the spectrochemical laboratory. Each portion was analyzed using the pellet-fusion-spark method. Three fusions from each portion were made. Duplicate exposures of one pellet made from each fused bead were used to obtain the results. The maximum deviation of the analysis of any one split-out portion from the mathematical average analysis of the four portions is less than 6 percent.
Coefficients of variation (v) were determined using the values for the individual fusions shown in table 11. These data, together with other precision data on the spark and arc methods, are shown in table 12.


Discussion and Conclusions
Time requirements for the two methods described are considerably different. One operator can make 18 beryllium determinations in one 8-hour day by the arc method; however, only 8 determinations can be performed in one 8-hour day by the fusion-pellet-spark procedure. The difference in determinations is due to the time necessary for preparing the fused bead, allowing it to cool, crushing, screening, and pelletizing. The stated times include requirements for sample preparation, weighings, exposures of samples and standards for one concentration range, plate processing, line measurements, and calculations.
If analyses were performed with direct reading equipment, thus eliminating the photographic process, the times required for analyses would be reduced appreciably and precision and accuracy may be improved.

where
x = average concentration in percent,
d = difference of the determination from the mean,
and
n = number of determinations.
Exposures for the calculation of the coefficients of variations in each case were made on 3 plates on different days.
Volatilization behavior curves for the arc procedure show that the vanadium internal standard and beryllium do not volatilize at the same rate; however, at varying concentrations of beryllium the volatilization patterns are similar. By using a total arcing time of 90 seconds, intensity ratios are obtained suitable for establishing the method. The use of some element other than vanadium as internal standard, which shows a volatilization pattern more nearly like that for beryllium, would be desirable. Also, the need to use atom-ion line pairs could probably be circumvented by employing some other element. For the spark procedure, the nearly parallel volatilization behavior of vanadium and beryllium shows that this requirement for internal standardization has been achieved. Excitation potentials are reasonably closely matched except in the highest concentration range where it was necessary to use an atom-ion line pair.
Effects due to matrix variations in samples and standards were eliminated in each procedure–in the arc method by dilution in germanium metal and graphite and in the spark procedure by fusion with lithium tetraborate. Wide variations in matrix composition of the mineral test products analyzed caused no difficulty.
Either of the two methods described is satisfactory for determining beryllium content of siliceous mineral test products and in silicate rocks. The accuracy and precision achieved with the fusion-spark-procedure is superior to that obtained with the arc method; however, where time requirements dictate that a faster method be used, the arc procedure can be employed with an accompanying sacrifice in precision and accuracy.

