Table of Contents
A spectrophotometry method for the determination of small amounts of copper in tungsten metal powder using the principle of solvent extraction has been developed. The metallic sample is dissolved in hydrogen peroxide, tartaric acid added, the pH adjusted, and the excess peroxide destroyed by boiling. Copper is reduced to the cuprous state, and the reagent neocuproine (2,9-dimethyl-1,10-phenanthroline) is added to form a colored complex, with the copper. This colored complex is then extracted into chloroform and the absorbance of the separated chloroform layer measured on a spectrophotometer. Copper contents as low as 1 p.p.m. (parts per million) can be determined. The method is limited to finely divided tungsten powder.
The interest in developing more sensitive methods for the determination of trace impurities in high-purity tungsten prompted an investigation of the possibility of using solvent extraction-colorimetric methods. The organic reagents now commercially available make possible many extremely sensitive colorimetric methods for the determination of metallic ions. Extraction of the colored complex into a small volume of organic phase can be used to further increase sensitivity. This general approach has the added advantage that no special equipment beyond that normally found in an analytical laboratory is required.
Previous experience in this laboratory Indicated the possibility of determining a few parts per million of copper in tungsten by a solvent extraction-colorimetric technique. The following investigation was undertaken toward development of this technique.
The reagent neocuproine was introduced by engineers, who recommended extraction of the colored complex in isoamyl alcohol. Gahler substituted chloroform for the extracting medium. Since the chloroform phase has a greater specific gravity than the aqueous phase, it collects in the lower portion of the separatory funnel and facilitates the separation. Luke and Campbell thoroughly tested the procedure for interferences and found no interfering ions other than sulfide and cyanide. Neither of these is involved in the present procedure.
The use of hydrogen peroxide to dissolve the samples was found to be advantageous in keeping the reagent blank to a minimum.
Reagents and Apparatus
All reagents were of analytical grade. Blank determinations run on all reagents resulted in absorbance readings representing 7 µg. of copper.
Hydrogen peroxide…………………………………..30 percent.
Tartaric acid…………………………….Analytical grade, crystal.
Sodium hydroxide………………………….20-percent solution.
Sulfuric acid……………………………………………1:1 by volume.
Hydroxylamine hydrochloride…………..5-percent solution.
Sodium citrate……………………………….30-percent solution.
Neocuproine………………….0.1-percent solution in ethanol.
Chloroform…………………………………………Analytical grade.
A stock copper solution was prepared by dissolving electrolytic copper foil in the minimum required quantity of nitric acid. A working solution containing 0.01 mg. of copper per milliliter was prepared from this stock solution by dilution.
A Beckman Model DU Spectrophotometer using 1 cm. cells was used in taking all absorbance readings. Any other spectrophotometer adjusted to 457 mµ should be satisfactory. Since the absorption curve of the colored complex has only one rather broad peak in the range 300 to 600 mµ, a filter colorimeter should also prove satisfactory.
Procedure
- Weigh a 10-g. portion of the tungsten metal powder sample into a 400-ml. beaker.
- Add about 10 ml. of distilled water. Cautiously add 30 pct. hydrogen peroxide, starting with small (5 ml.) portions. The reaction between tungsten metal powder and 30-pct. hydrogen peroxide is exothermic and tends to accelerate as it warms. The reaction can be slowed, when necessary, by immersing the beaker in a shallow water bath. Continue adding 30-pct. hydrogen peroxide in gradually increased quantities until the sample is dissolved and the solution is clear and colorless. Approximately 90 ml. of hydrogen peroxide is required. Near the end of the reaction, moderate warming of the beaker on a hotplate may be necessary.
- Add 10 g. of solid tartaric acid.
- Neutralize the sample solution with 20-pct. sodium hydroxide solution.
- Add 1:1 sulfuric acid to adjust the solution to pH 4.
- Boil the solution for 30 min, to remove excess hydrogen peroxide.
- Cool the solution and transfer it to a 250-ml. separatory funnel.
- Add the following reagents, mixing well after each addition:
(a) 10 ml. of 5-pct. hydroxylamine hydrochloride solution.
(b) 10 ml. of 30-pct. sodium citrate solution.
(c) 10 ml. of 0.1-pct. neocuproine solution.
(d) 10 ml. of chloroform. - Shake the separatory funnel for i min., then allow it to stand until the chloroform layer separates. Drain the chloroform layer into a 25-ml. volumetric flask containing approximately 5 ml. of ethyl alcohol.
- Add 5 ml. of chloroform to the separatory funnel, shake for 30 seconds, allow the layers to separate, and add the chloroform layer to the previous portion in the volumetric flask.
- Dilute the combined chloroform layers to 25 ml. with ethyl alcohol and mix thoroughly.
- Read the absorbance of the chloroform solution against distilled water at 457 mµ. Correct this reading for a reagent blank carried through the entire procedure. Calculate the percentage of copper by a calibration curve, prepared by using known amounts of copper.
Experimental Results and Conclusions
Small quantities of copper are known to be quantitatively extracted from dilute aqueous solutions by the neocuproine-chloroform extraction procedure. The absorbances of extracted chloroform phases have a linear relationship to quantity of copper present. Experiments were designed to determine whether the quantitative extraction and linear relationship applied to solutions containing large quantities of tungsten.
Since no standard sample of tungsten with a copper content in the parts-per-million range was available, it was necessary to use the following approach:
- 10-g. portions of tungsten having a copper content of less than 10 p.p.m. by spectrographic analysis were treated according to the above procedure. The results are given in table 1.
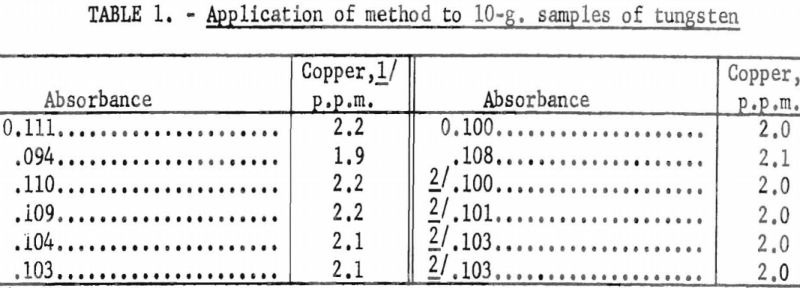
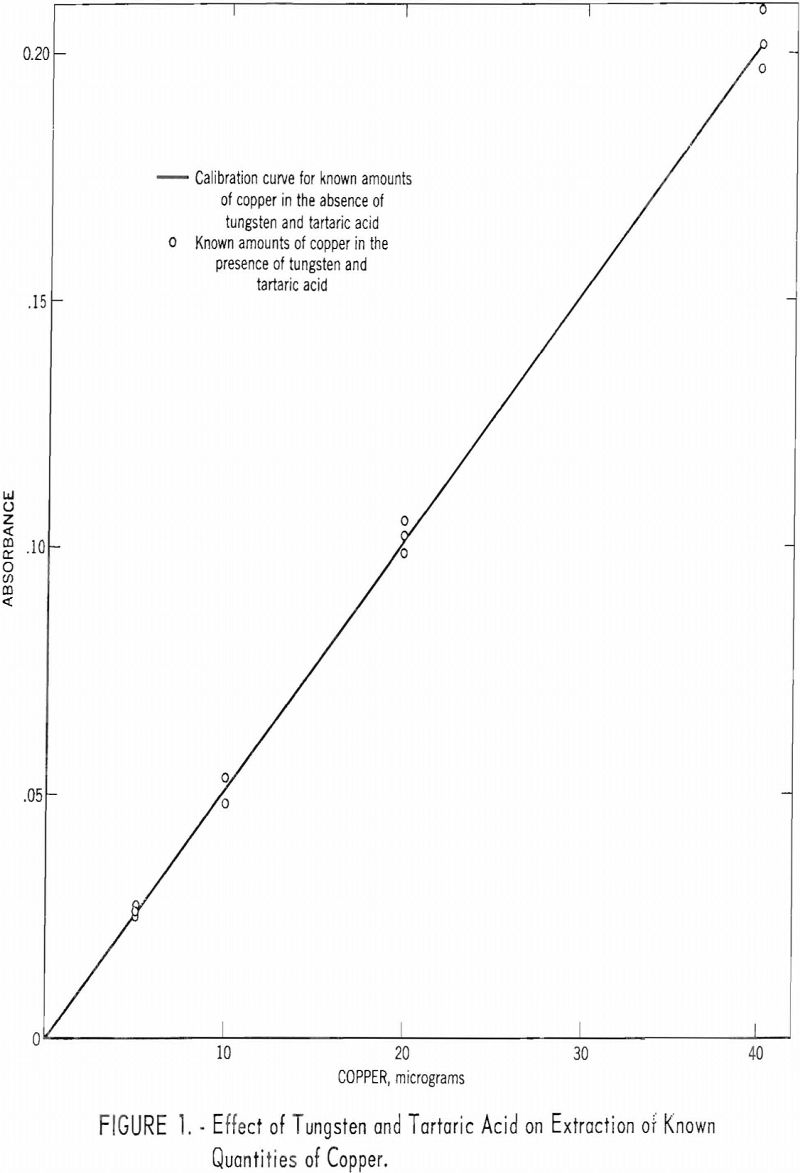
- Known amounts of copper were added to the aqueous phase remaining from the above tests and steps 8 through 12 were repeated. These results were plotted on a calibration curve previously made using known amounts of copper in the absence of tungsten, tartaric acid, and hydrogen peroxide. The results are given in table 2 and shown in figure 1.
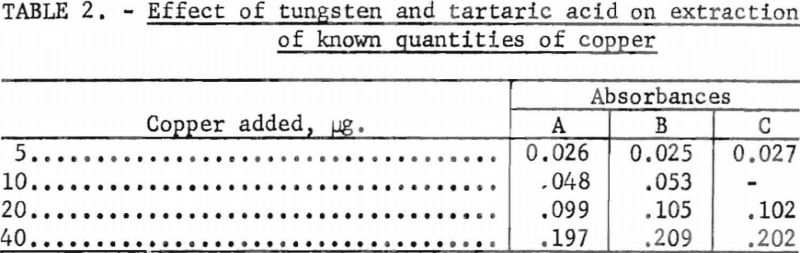
These results indicate that small quantities of copper are extractable from solutions containing large quantities of tungsten.
The fall of these points on the previous calibration curve indicates that the presence of 10 g. of tungsten, 10 g. of tartaric acid, and any hydrogen peroxide remaining from the solution procedure have no effect on the relationship between the absorbance of the chloroform extract and the quantity of copper present.
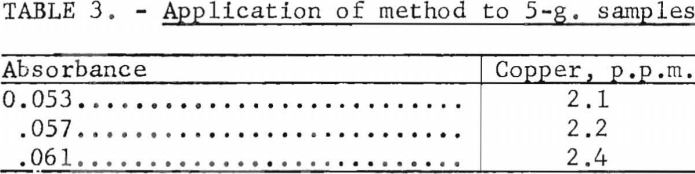
These tests would not indicate whether a constant quantity of copper failed to be extracted in the presence of tungsten and tartaric acid. If such a tendency toward incomplete extraction existed, decreasing the sample weight could cause a decrease in the recovery ratio. Therefore, 5-g. of the tungsten metal powder were treated by the same procedure. The results are given in table 3.
Since these experimental results are essentially the same as those obtained on 10-g. samples, it can be assumed that extraction is complete.
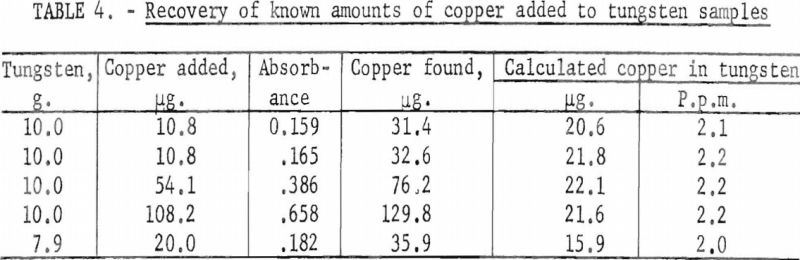
As a further test of the method, known amounts of copper were added to solutions of tungsten metal powder taken into solution, as described in this procedure. The results are demonstrated in table 4.
The results indicate that the neocuproine-chloroform-extraction colorimetric method can be used to determine copper concentrations in the few parts-per-million range in tungsten metal powder.
All the above tests were performed on tungsten metal powder of 0.002- to 0.005 mm. particle size. Attempts to apply the same procedure to material of almost identical chemical composition but of 0.2- to 0.5-mm. particle size produced low and inconsistent results. Adding more hydroxylamine hydrochloride end repeating the extraction produced more of the colored copper complex. It is therefore believed that the difficulties are caused by the presence of a higher oxidation state of tungsten (possible perditungstate) in the solution produced from the material of larger particle size. This is further indicated by the fact that yellow tungstic oxide is produced more readily in solutions prepared from the material of finer particle size than from solutions prepared from the coarser material on boiling. The decreased surface area of the larger particles could well cause sufficient decrease in the rate of reaction between hydrogen peroxide and the metal and consequently favor the reaction of hydrogen peroxide on the tungstic acid already formed.
