Table of Contents
Supercritical water alters the structure of common sulfide minerals by partially removing sulfur from the mineral structure. This discovery has been applied to the treatment of pyritic gold ores to improve precious metal recovery without generating or utilizing environmentally sensitive products such as SO2, chlorine, or cyanide. Pretreating pyrite concentrates in supercritical water under mildly oxidizing conditions provided by certain metal oxides or oxygen improved gold leaching using thiourea. The pretreatment differs from chlorination or roast pretreatments in that elemental sulfur rather than sulfuric acid is the major product. A pyritic ore containing 58 g/mt Au was pretreated with oxygenated supercritical water at 24 MPa and 400°C, then leached with 20 g/L thiourea. With pretreatment, gold recovery was 90%; by contrast, gold recovery was below 10% without pretreatment.
Recent work at the Bureau of Mines showed some unique and potentially useful results when refractory gold ores were pretreated with supercritical water before standard leaching. This work is described below. Because the work was exploratory, this paper is a status report; much more research is needed to judge whether the technology is commercially feasible.
Supercritical fluids possess unique properties which differ markedly from fluid properties at ambient temperatures and pressures. Differences between properties of liquid and gas phases diminish as a substance approaches the critical point. At the critical point, all differences disappear and the phases become mutually soluble. These transformations are indicated in Figure 1 by the termination of the vapor-liquid curve. Commonly, properties of the supercritical phase are intermediate to the properties of the liquid and gas. Supercritical water, however, exhibits even greater changes from the liquid state because thermal energy exceeds the strength of water’s hydrogen bond. As a result, ionized species become much less soluble, while hydrocarbons become much more soluble.
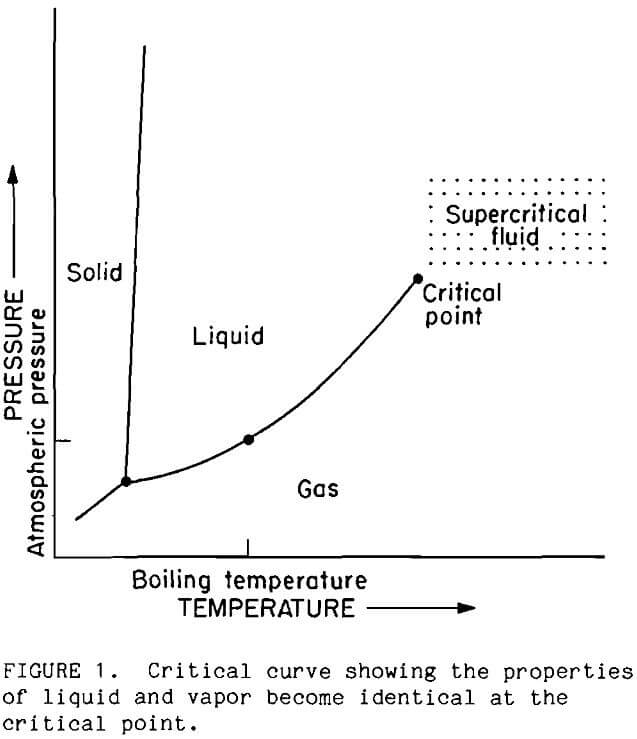
In recent years the amount of research on supercritical phenomena has increased rapidly (Paulaitis, 1983), and commercial processes using supercritical fluids are now being adopted by industry. The most widely recognized supercritical process is coffee decaffeination that is advertized as using only pure water and natural effervescence. In the areas of minerals and earth sciences, geologists are doing extensive investigations on mineral solubilities in water at high temperatures and pressures; the data are used in modeling the formation of ore deposits (Morey, 1957; Franck, 1970; Marshal, 1985; McKenzie, 1984; Frantz, 1981). Oceanographers are finding sulfide mineral deposits around hydrothermal vents in the ocean floor (Edmond, 1983). Apparently, ocean water seeps into hot rock formations below the ocean floor and is heated to near-critical or supercritical temperature. The combination of temperature and pressure causes dissolution of minerals. The solutions emerge through vents in the ocean floor. Pyrite is the most abundant sulfide mineral in the vent water. Bureau researchers discovered that several common minerals interact in supercritical water (Oestreich, 1989; Isaacson, 1989). For example, pyrite was observed to decompose to hematite and sulfur in the presence of copper oxide minerals.
Bureau researchers attempted to apply this ore dissolution chemistry as a method to improve metal recoveries from complex or refractory ores. Pyrite is known to inhibit gold leaching (Nicol, 1987); supercritical water pretreatment was tried as a method of improving gold recovery from auriferous pyrite ore while yielding innocuous sulfur as a byproduct.
Another common inhibitor of gold leaching is indigenous organic carbon in the ore. This material acts much the same as activated carbon in absorbing the gold cyanide complex from solution. The net result is that the gold is irretrievably locked into the ore and is lost in the tails. Little is known about the composition and chemistry of the organic material. This lack of knowledge has impeded efforts to remove the active absorbent. Qualitatively, the carbonaceous absorbent behaves much like naturally absorbent peat, lignite, or coal. Several literature reports (Berkowitz, 1987; Deshpande, 1984; Kershaw, 1989) have discussed coal, lignite and peat gasification with supercritical water, suggesting the possibility of removing the carbonaceous matter from gold ores using supercritical water. Potentially, then, supercritical water might eliminate simultaneously both carbonaceous material and sulfides that cause refractory behavior in gold ores.
Pretreatment with supercritical water may have distinct advantages.
- The major reagent is water, so corrosive, toxic, or hazardous feed materials are avoided;
- Pyritic ores yield elemental sulfur rather than sulfuric acid as a major product, potentially reducing acid neutralization requirements of the sulfide removal process;
- Many organic compounds become soluble in supercritical water; preg-robbing carbonaceous matter in gold ore may dissolve in supercritical water and be removed in the pretreatment.
The tradeoff is that supercritical water pretreatment operates at 24 MPa, which is higher than pressures commonly used in mineral processing. This report discusses results of preliminary supercritical fluid tests to improve gold recovery from refractory ores.
Experimental Procedures
Description of Gold Samples
Two ore samples were obtained from a Nevada gold operator. These were hand picked from drill core samples. No further concentration was attempted. One sample, designated “pyritic,” contained 5% pyrite in a quartz matrix and assayed 58 g/mt Au. Leaching this material with either cyanide or thiourea consistently yielded less than 10% gold extraction. No attempt was made to determine whether the gold occurred on the surface of pyrite grains or was incorporated in the mineral matrix. The ore was crushed to less than 200 mesh for testing. The second sample, termed here “carbonaceous” for brevity, contained 5% pyrite and 0.79% organic carbon in a quartz matrix and assayed 5.5 g/mt. Previous cyanide leaching (5 g/L NaCN at pH 10.5 to 11 for 5 h) by the supplier extracted no gold from this ore. The ore was sized to 65 x 200 mesh to remove slimes that blocked flow of supercritical water through the ore; sizing had no measurable effect on leaching.
Two flotation concentrates also were tested. One pyrite flotation concentrate was obtained from a mine in California. The concentrate assayed 57.5 g/mt Au. Gold leached readily from this concentrate without a pretreatment. A pyritic ore was obtained from a mine in Alaska. The ore was crushed and concentrated by flotation to obtain a sample that assayed 3-8 g/mt Au. This concentrate responded poorly to leaching.
Supercritical Water Apparatus
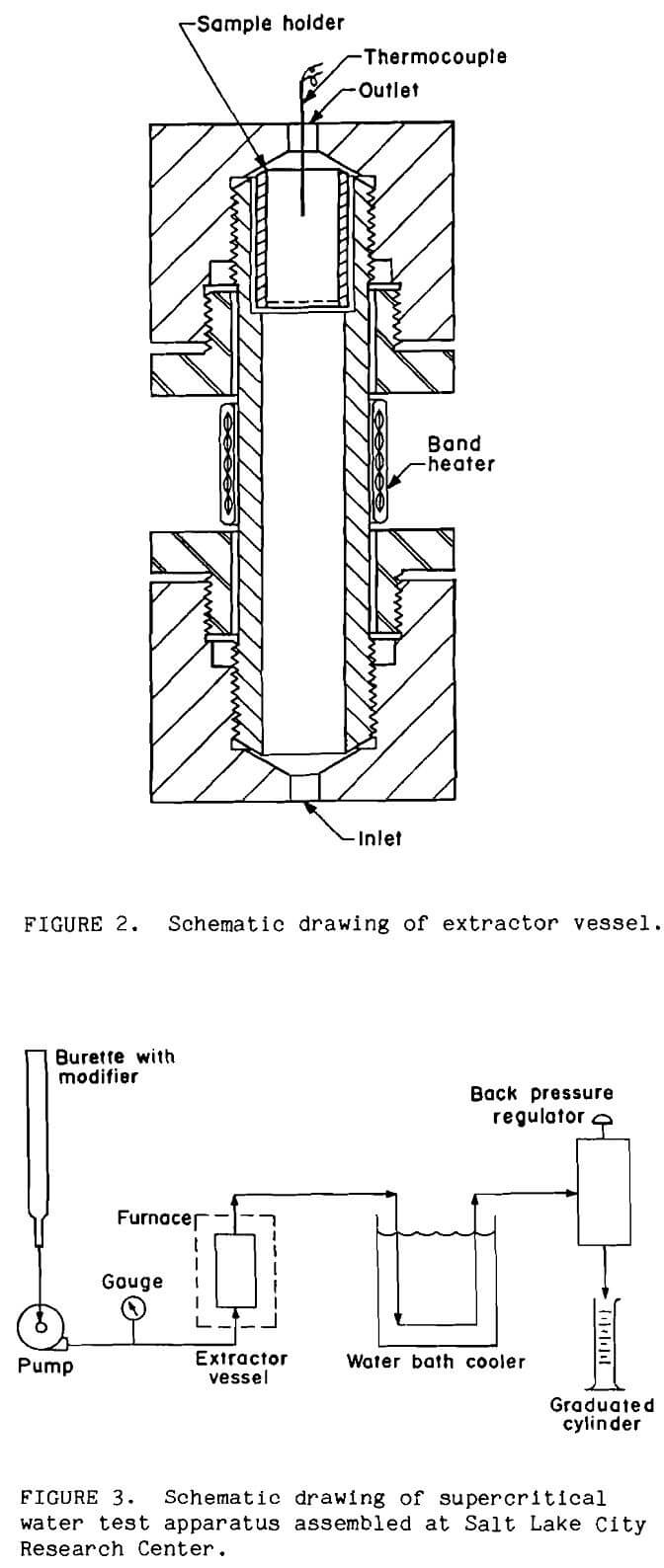
The ores were pretreated in a batch-continuous system developed by the Bureau for treating metallurgical samples in supercritical water (Tolley, 1986). Ore samples were held in the extractor vessel as shown in Figure 2. This was a 152-mm long by 25-mm OD 316 stainless steel pipe nipple. Tubing connected to the extractor vessel with coned fittings. A 60µm stainless-steel, fritted filter element or stainless-steel wire mesh retained the sample in the extractor vessel. The extractor vessel was held vertically in a muffle furnace and operated in an upflow mode. The furnace was held slightly below the target extraction temperature to prevent unnecessary thermal stress on the fittings; a band heater was wrapped around the vessel to attain the final temperature inside the vessel of 400°C. A thermocouple inserted through the top of the extractor vessel monitored sample temperature.
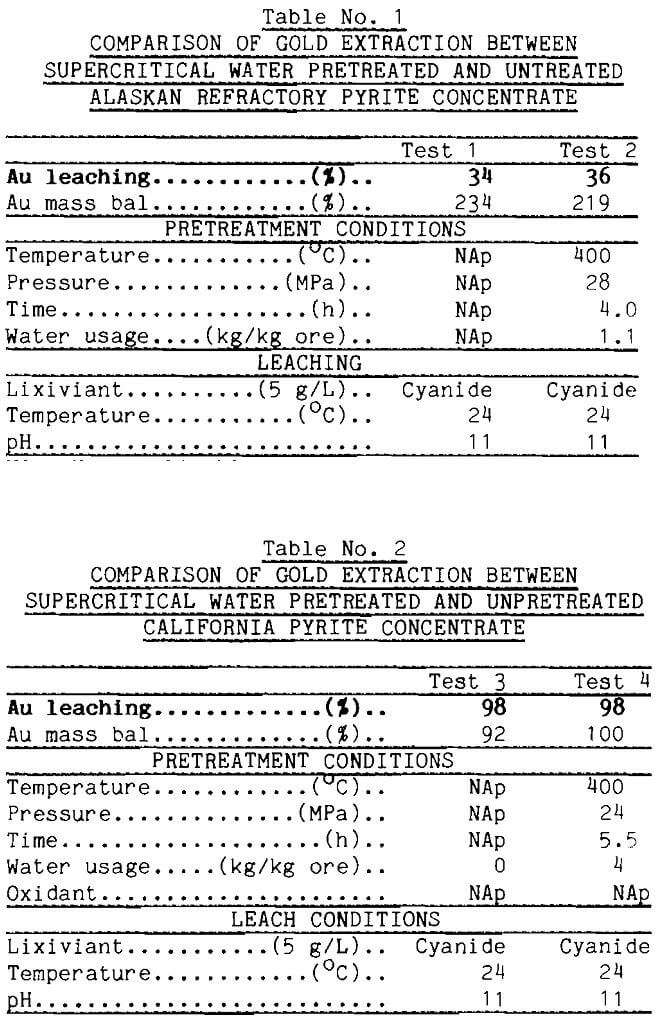
A schematic of the test apparatus is shown in Figure 3. Basic material of construction for the system (Tolley, 1986; Oestreich, 1989; Isaacson,
1989) was 316 stainless-steel tubing. Feed water was purged under slight pressure with either argon or oxygen depending on the desired oxidation potential in the pretreatment. Water flowed by gravity from the feed burette to a Lewa diaphragm pump, which could produce 70 MPa pressure. Pressure was adjusted with a Tescom back pressure regulator connected to the exit of the extractor vessel.
Procedures
Supercritical Fluid Pretreatment Mineral samples were pretreated batchwise in the supercritical apparatus. The vessel held approximately 25 g of mineral in each batch. The system was purged with water at atmospheric temperature and pressure to displace atmospheric oxygen. The back pressure regulator then was reset for the desired operating pressure and heating was initiated. Standard pretreatment conditions were 400°C and 24 MPa for 5.5 h with a flow rate at 0.3 mL/min water. Water usage was approximately 4 kg/kg ore, or 15 bed volumes. The pump continuously forced liquid water into a preheater during the tests to produce flow conditions within the ore sample. The extract exiting through the back pressure regulator was collected in a graduated cylinder. The extract liquid was analyzed for pH and dissolved solids.
After the pretreatment period was completed, the system was allowed to cool to ambient temperature. Products were removed from the apparatus, rinsed with acetone, and dried under reduced pressure.
Leaching The standard thiourea leach was 20 g/L thiourea in dilute (pH 1) H2SO4 for 5 h. The oxidation potential during leaching was maintained above 200 mV measured against silver chloride reference. Pulp density was 24.0% (3 L solution/kg solids, 60 g thiourea/kg solids). The standard cyanide leach conditions for the pyritic ore were 5 g/L NaCN in pH 11 NaOH for 5 h (3 L solution/kg solids, 15 g NaCN/kg solids). Cyanide solutions used in leaching the carbonaceous ore were dosed with 10 mg/L Au to assess the absorptive capacity of carbonaceous residues. The objective of the supercritical pretreatment was to deactivate the carbonaceous material and allow the cyanide to leach the gold from the ore. If this occurred, the gold level in solution would increase as gold dissolved out of the ore. Conversely, the pretreatment could prove ineffective and gold could disappear from solution by absorption onto the carbonaceous matter. Because the carbon was highly absorptive, the pulp density was changed to 2% (50 L solution/kg solids). Under these conditions, the gold in the ore accounted for only 1$ of the gold in the system.
The solids were analyzed by fire assay and X-ray diffraction. Liquids were analyzed by fire assay, ICP, and AAS.
Results and Discussion
Pyritic Gold Concentrates
The data show that supercritical water pretreatment made the refractory pyritic ore amenable to high gold recovery using standard leaching methods. Leaching achieved 90% gold recovery after a controlled-oxygen pretreatment in supercritical water. In contrast, leaching yielded only 9% gold recovery without the pretreatment.
Bureau of Mines researchers have described mineral behavior in supercritical water (Oestreich, 1989; Isaacson, 1989). One observation was that iron sulfide minerals usually showed alteration. The iron converted to hematite. Simultaneously, elemental sulfur was released into the water. The extent of reaction depended on the oxidation potential of the solution.
These observations suggested that supercritical water could remove sulfur from pyrite, thereby making refractory pyritic ores amenable to leaching. The hypothesis was that supercritical water at an appropriate oxidation potential would remove the leach-inhibiting sulfur from auriferous pyrites. Controllable parameters in this treatment included oxidation potential, pH, and the ratio of water to ore. One benefit anticipated from this technique compared with traditional autoclaving was that the sulfur was recovered primarily in the elemental state; thus, one need not neutralize copious amounts of sulfuric acid that would otherwise be formed.
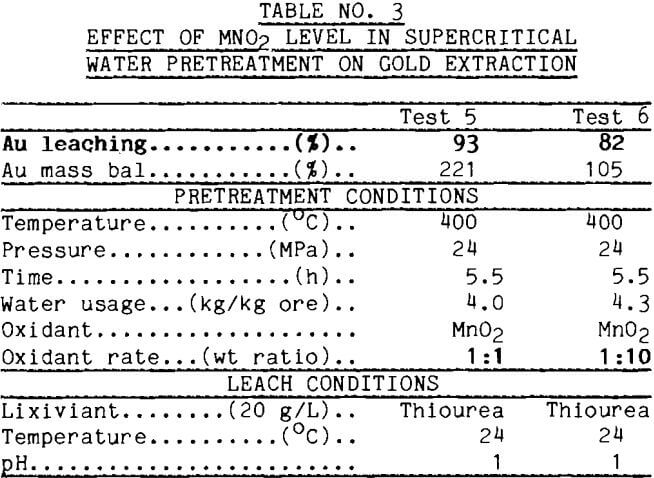
Early tests were conducted to assure that heating in supercritical water alone had no effect on leach response. To test this, the Alaskan pyrite concentrate was heated to 380°C with sufficient water to maintain pressure. Pretreated and untreated ore then were leached with cyanide in parallel tests. Results in Table 1 show that no benefit was derived from merely heating the ore in supercritical water.
Another test was conducted to determine that heating in supercritical water did not decrease gold leaching measurably. The concentrate from California, which leached readily with no pretreatment, was tested in the supercritical apparatus, then leached. The results of this test along with data from a leach of untreated concentrate are reported in Table 2. The data show no deleterious effect from supercritical water pretreatment.
The pyritic ore proved to be highly refractory. Leaching the crushed, untreated ore with 20 g/L thiourea for 5 h extracted only 9% of the gold. The Nevada operator reported comparable results using cyanide leaching.
Metal Oxides in the Supercritical Water Pretreatment
Manganese Dioxide Several reagents were chosen to change the oxidation potential in supercritical water, including manganese dioxide, cupric oxide, and oxygen. In preliminary tests, the ore was mixed with MnO2 and treated in a standard supercritical water test. MnO2 was added to the ore in weight ratios of 1:1 and 1:10. After supercritical water pretreatment, the ore sample was leached with thiourea. Gold extraction shown in Table 3 was 93% from the 1:1 product and 82% from the 1:10 product. The large error in the mass balance of test 5 suggests that these extractions are equivalent. The significant point is that pretreatment with either level of MnO2 yields improved leaching over the 9% gold recovery without pretreatment.
These data point out that controlled oxidation enhanced the gold leaching. The oxidation mechanism is not clear, but a major product was colloidal sulfur rather than a more oxidized form such as sulfuric acid. X-ray fluorescence and diffraction confirmed that the material was crude sulfur. The sulfur precipitated from the aqueous effluent leaving the supercritical extractor. The effluents generally ranged from pH 4 to pH 6. These were analyzed for gold, but none was detected.
Temperature Effects: Temperature proved to be a significant parameter as the reagent addition rate was decreased. Portions of the ore/MnO2 mixtures just described were tested at subcritical (<374°C) conditions of 250°C and 24 MPa. This temperature was low enough to suppress most of the reactions observed in earlier mineral tests (Oestreich, 1989); the pressure was chosen to be consistent with the previous tests. For the 1:1 mixture shown in Table 4, gold extraction after the 250°C pretreatment was 94%; lowering the temperature had not affected gold extraction. For the 1:10 mixture, temperature became important and only 58$ of the gold was extracted during thiourea leaching. Thus, it becomes an economic issue whether higher reagent addition or higher pretreatment temperature is used to raise the gold extraction.
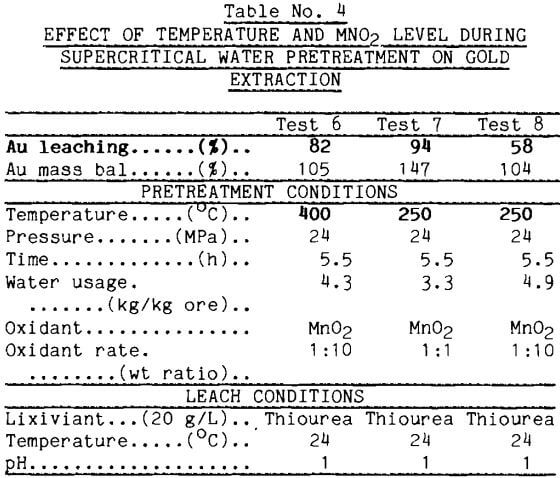
The action of MnO2 on sulfides to produce element sulfur has precedent in metallurgical practice. Common metal sulfides react with MnO2 in sulfuric acid solutions to produce elemental sulfur (Sandberg, 1979). Supercritical water dissolves this sulfur product and carries it away from the gold ore.
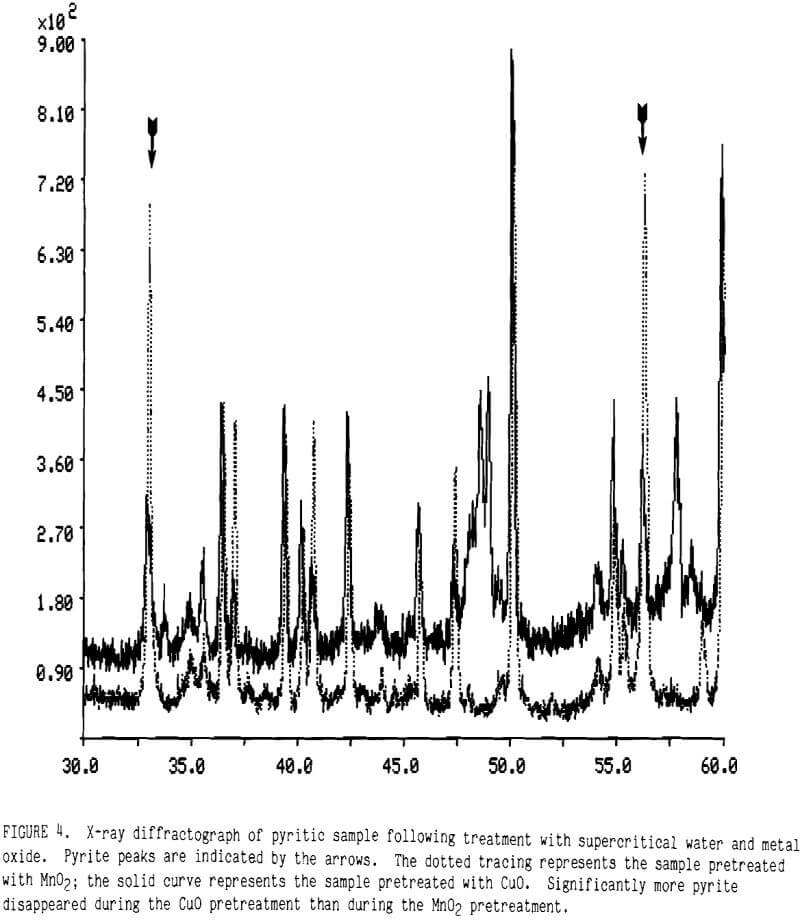
Cupric Oxide While MnO2 was effective in raising gold extraction, it was inefficient because the MnO2 ended up as Mn5O8 and Mn2O3 rather than Mn(II). Copper oxides in supercritical water appeared to react much more completely with iron sulfides than did the manganese dioxide. This is demonstrated from the X-ray diffraction data plotted in Figure 4. The strong peaks for pyrite, indicated by arrows in the figure, remain much more intense after pretreatment with MnO2 (the dotted tracing) than after pretreatment with CuO (the solid tracing). Although MnO2 is a stronger oxidant than CuO, the affinity of copper for the sulfur in pyrite made CuO more effective in decomposing pyrite.
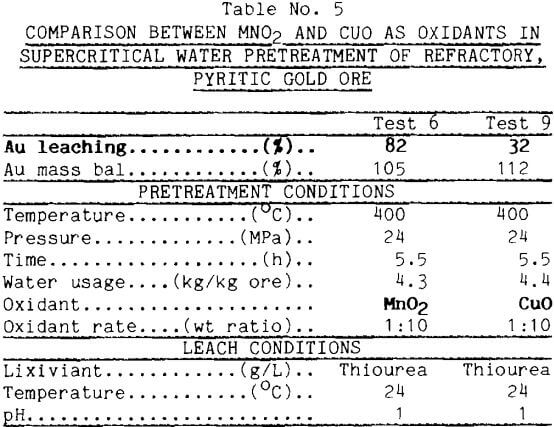
Thiourea leaching after CuO pretreatment, however, yielded less gold. As seen from the data in Table 5, supercritical pretreatment with CuO produced only 32% Au leaching contrasted against 82% using MnO2. This result emphasizes that complete destruction of the pyrite mineral is not necessary to obtain high gold leaching.
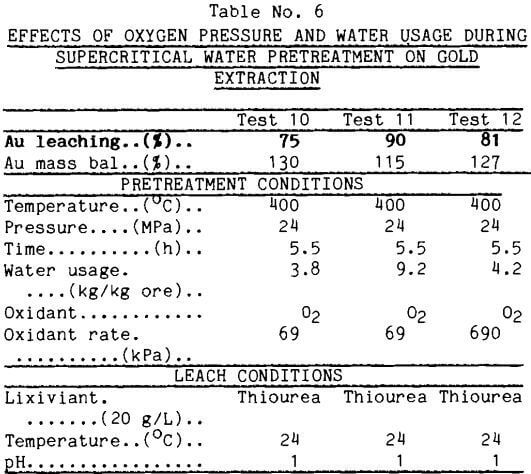
Oxygen as the Oxidant Another readily available oxidant is O2 gas. The feed water to the supercritical vessel was saturated with O2 at several pressures. During the supercritical water pretreatment, colloidal sulfur again precipitated from the effluent of the supercritical reactor. Gold was not detected in the effluents.
Thiourea Leaches: The data in Table 6 show gold recovery in thiourea leaches after supercritical water pretreatment. Oxygen in the supercritical water proved very effective in enhancing gold recovery by thiourea leaching. These data also indicate a synergism in the action of water and oxygen; thus, more oxygen pressure alone did little to improve gold leaching. Test 12, with the higher O2 pressure, yielded less gold upon leaching than test 11, which received more water at the lower oxygen pressure. There are insufficient data to propose an explanation.
Cyanide Leaches: Since cyanide leaches are the industry standard, samples of the pretreated ore were leached with a cyanide solution (5 g/L NaCN) rather than thiourea. Within experimental error, supercritical water pretreatment worked equally well with either a thiourea or a cyanide leach, as shown in Table 7.
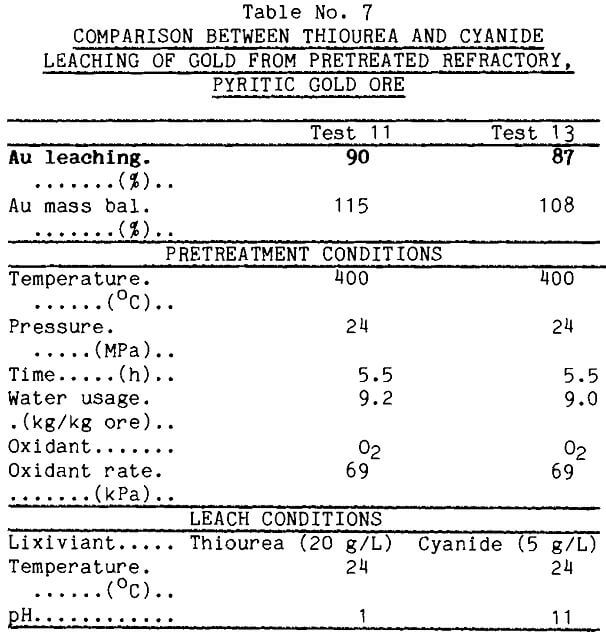
Effect of Temperature on Low-Oxygen Pretreatment To determine the effect of temperature in combination with oxygen, a pretreatment was run at 300°C keeping time, oxygen pressure, water flow rate, and leaching parameters constant. Data from Table 8 show that thiourea leached just over half of the gold from this sample contrasted with the 90% recovered after the 400°C pretreatment. X-ray diffraction of the leach tails showed subtle differences in the siliceous ore component between the material treated at 400° and that treated at 300°, but this is not believed to be influencing the gold extraction.
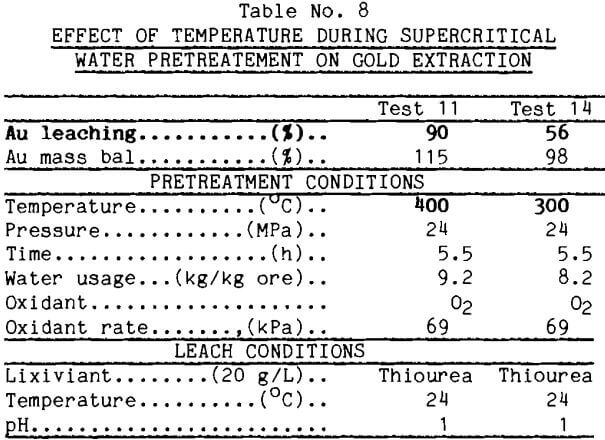
The supercritical water tests normally were run for 5.5 h. It was desirable to reduce this time. Because of limitations in the supercritical apparatus, the shortest practical operating time under controlled conditions was 1.25 h. This time was used in test 15 reported in Table 9. This pretreatment enhanced gold leaching well as long as enough water was used. The rapidity of the pretreatment also was demonstrated in another test (test 16) which was unexpectedly shortened when the furnace elements arced and failed. Gold extraction from the pretreated material, however, was good.
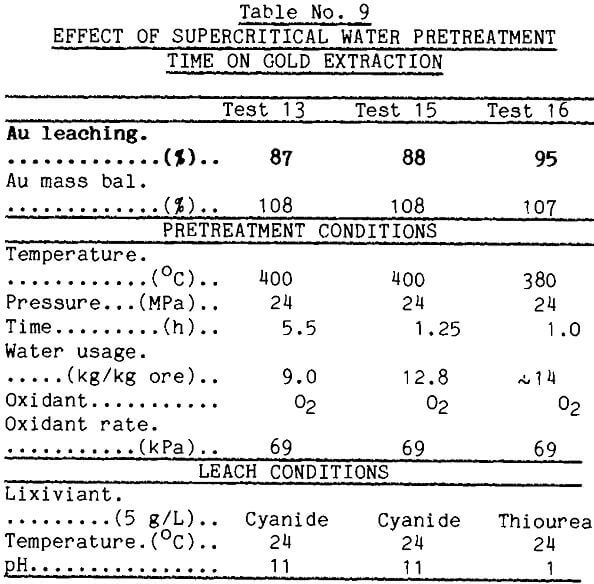
These data show that temperature, oxygen level, and volume of water pumped through the ore are critical. The best gold extractions were obtained when the ore was pretreated with low levels of oxygen, but the optimum oxygen is not known. Pretreatment time in this investigation was limited by the physical parameters of the system rather then chemical kinetics of the mineral alteration. Pressure effects have not been studied.
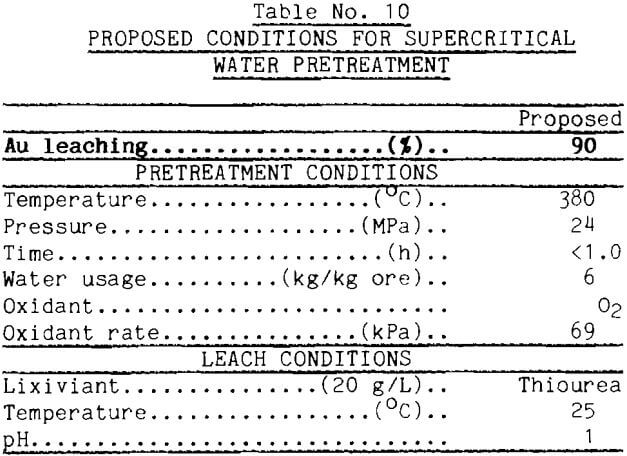
Proposed operating conditions for supercritical water pretreatment of refractory pyritic ore based on the best gold extractions found so far are presented in Table 10. The temperature needs to be above the critical temperature; 380°C probably is adequate. Water usage needs to be greater than 3 parts water for each part of ore; 6 to 7 parts water appears sufficient. Oxygen at a partial pressure of approximately 69 kPa in the feed water worked well in these tests. Time does not need to be longer than about an hour, and may be less if good heat and mass transfer are built into the supercritical system. The table specifies a thiourea leach, but cyanide worked well in this investigation. How the supercritical water pretreatment frees the gold for leaching is poorly understood.
Organic Carbon in Gold Ore Another common cause of gold loss is organic carbon naturally occurring in the ore. In the sulfidic- carbonaceous ore, carbonaceous material acts much the same as activated carbon in absorbing the gold cyanide complex from solution. The net result is that the gold is irretrievably locked into the ore and is lost in the tails. This effect was observed by adding 10 mg/L Au to the leach solution before the ore was added and measuring the loss of gold from solution. Because the ore was so active, 50 L of solution/kg ore were required to prevent complete absorption of gold from solution. From a solution containing 10 mg/L Au and 5 g/L NaCN, the carbonaceous ore absorbed 222 g/mt Au more than was in the ore originally, which was 55% of the gold in solution.
Because supercritical water solubilizes many organic materials, supercritical pretreatment was tried as a method for removing the absorbent from carbonaceous gold ores. Supercritical water, however, proved ineffective as seen from data for test 17 in Table 11. It should be noted, however, that water flow in these tests was very low. Even with sized ore, low bed porosity produced a high pressure drop (3.5 MPa) and prevented attainment of the desired water flows. This is unfortunate in view of the strong effect that water flow had in the earlier tests. Time limitations have prevented testing with more favorable water flows. Additions of modifiers such as H2SO4 or O2 to the supercritical water as in tests 18 and 19 produced the undesired result of raising the absorption capacity.
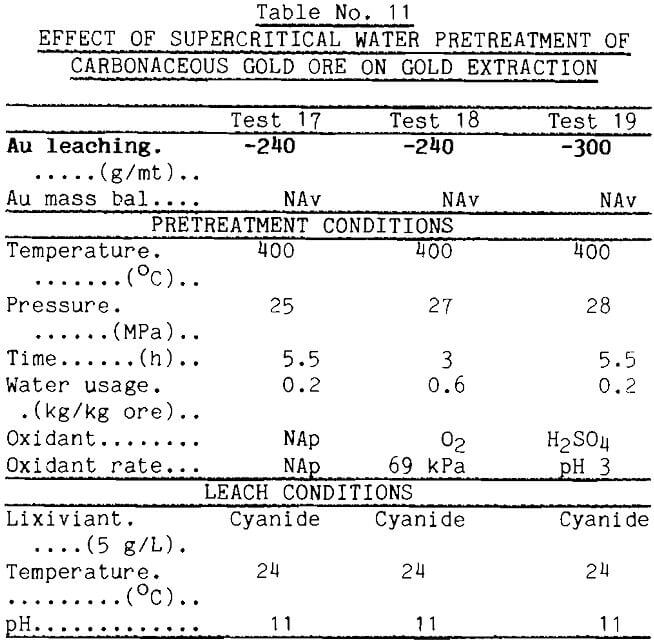
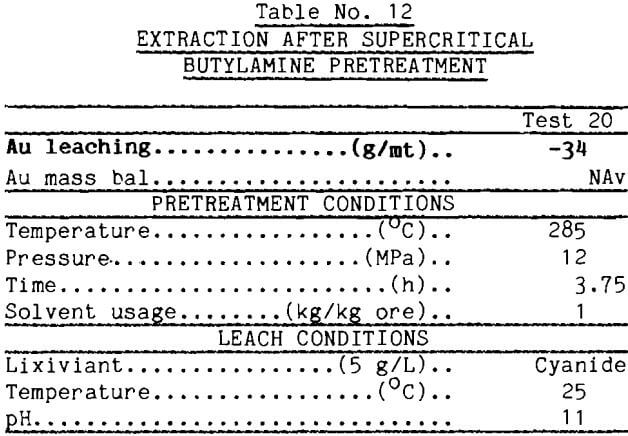
The concept of liquefaction of the carbonaceous matter appears to have merit, however. The literature contains reports showing that supercritical butylamine is highly effective in liquefying lignites (Chen, 1985). Accordingly, the carbonaceous gold ore was pretreated with supercritical butylamine at 285°C and 12 MPa, then leached with Au-dosed cyanide solution. The data reported in Table 12 show that the carbon absorbed only 45 g/mt gold from solution. Thus, 80% of the absorbent was deactivated by the buylamine pretreatment.
Conclusions
Supercritical water pretreatment has shown promise on a laboratory scale for improving gold recoveries from refractory auriferous pyrite ores. The pretreatment dramatically improved gold leaching from the ores tested here. This improvement occurred without major changes in the ore composition. Extrapolation from tests with pure pyrite indicates that the majority of the extracted sulfur was in the elemental form. Much research remains before pretreatment could be applied on a commercial scale. Many factors such as pressure and water recycle have not been examined.
Supercritical water pretreatment of refractory carbonaceous gold ores did not achieve the desired improvements in gold recovery. The concept of liquefaction to deactivate the carbonaceous absorbent in gold ores appeared to have merit, however. Developing a single, simple treatment that will handle both pyritic and carbonaceous types of refractory gold ores remains an enticing goal; supercritical solvent extraction may provide a way to achieve this goal.
