Table of Contents
For sulfide minerals, the presence of dissolved oxygen in the water phase is an influencing factor for separation by flotation, Klassen and Mokrousov distinguish three stages of oxygen adsorption; namely, reversible adsorption, activated adsorption and, finally, surface oxidation. While contraversial, limited adsorption of oxygen is believed to improve the interfacial reactivity between collector and mineral. Intensive surface oxidation of sulfide minerals usually results in unsatisfactory flotation with collectors of the xanthate type.
Products of Oxidation
Eadington and Prosser state that the principal products of oxidation of lead sulfide by oxygen dissolved in water are sulfur, basic lead thiosulfate, some polythionate and, ultimately, lead sulfate. These investigators believe that the reaction proceeds by at least four stages; (1) an induction period, the length of which depends on the non-stoichiometry of the lead sulfide, (2) a short acceleratory period, (3) a period of steady rate of oxidation, in which the rate depends on non-stoichiometry and irradiation, and, (4) a period of retardation by lead sulfate.
Sharply contrasting is the general lack of information with regard to the oxidation products on the surface of sulfide minerals. Leja, Little and Poling state that PbS2O3 is the original oxidation product present on the surface of lead sulfide, but rapidly decomposes to PbSO4. Also Eadington and Prosser recognized the presence of PbSO4 but also include elemental sulfur. For chalcocite, knowledge of the oxidation products at the mineral surface is notably lacking. Indeed, that the surface-oxidation products can have an effect on flotation is given notice. Plante states that: “Oxidation may affect flotation in two ways: (1) by alteration of the mineral surface with a consequent effect on adsorption of xanthate, and (2) by formation of oxidation products which may have depressant or activating effects.”
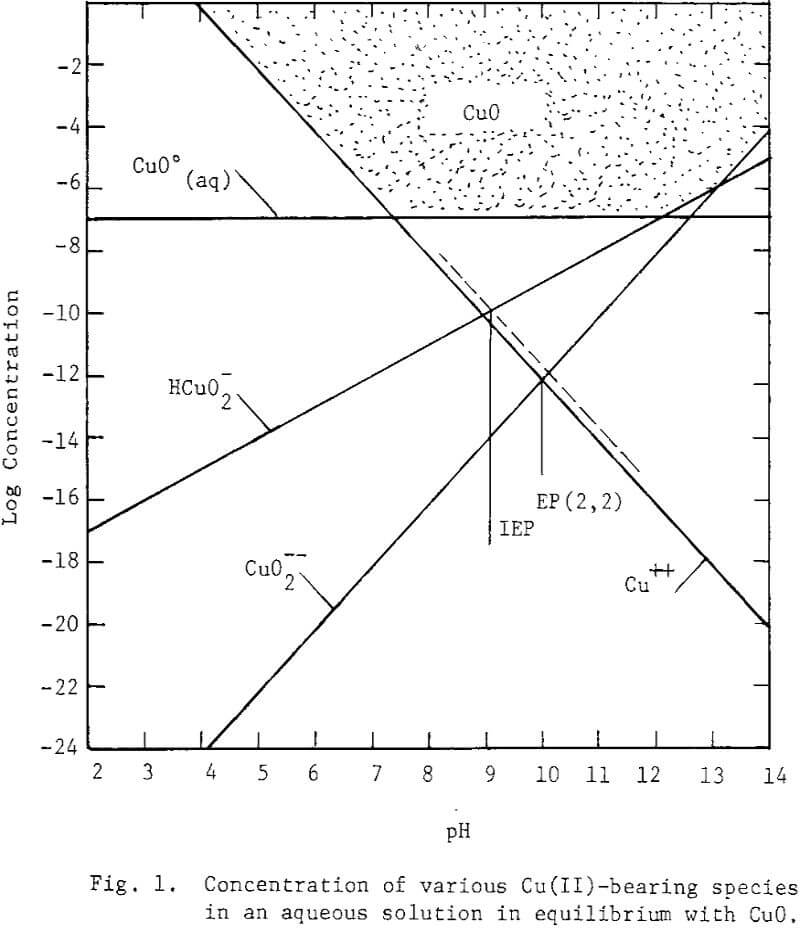
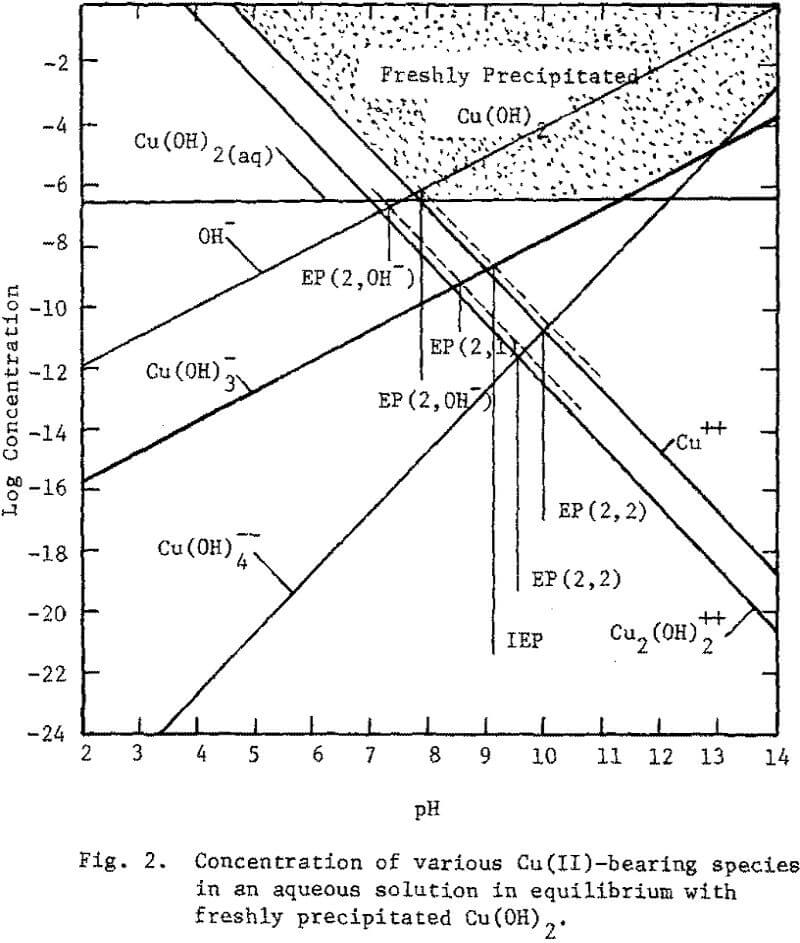
At the chalcocite interface the oxidation products of copper could be Cu(OH)2, CuO, Cu2O and Cu or ionic entities thereof. Thermodynamic analysis suggests the formation of CuO as the predominate specie at the interface. CuO, a neutral oxide, is stable Cu(OH)2, the neutral hydroxide, is metastable decomposing to CuO. From the hydrolysis of copper ions, equilibrium diagrams are presented in Figs. 1 and 2.
The predominating copper species in equilibrium with Cu(OH)2 are Cu++, Cu(OH)3- Cu (OH)4– and Cu2(OH)2++.
Experimental
Procedures were developed to oxidize particulate chalcocite; and, analytical instrumentation was used to identify the products of oxidation and to assess the effects of surface oxidation on flotation. Construction of a Eh-pH diagram for the system Cu-S-O2-H2O aided in the thermodynamic prediction of surface compounds.
Flotation tests were carried out on ‘fresh chalcocite’ and oxidized chalcocite. For the test of oxidized chalcocite, the procedure for oxidation was the same as previously described, except the particle size of chalcocite (-65/+325 mesh) was larger, 1000 mg of sample was used and the volume of water was less (100 ml). At the end of the oxidation period and immediately before each test, the water was drawn off without disturbing the settled particulates and the volume adjusted to 140 ml by addition of the required amount of collector and water. The collector used was purified sodium isopropyl xanthate (Aero Xanthate 343, American Cyanamid Company).
Experimental Results
Cupric oxide, CuO, is the oxidation product formed on the surface of chalcocite (compare spectrogram 4 – wavenumber 495 cm-¹. Surface CuO expectably retains a superficial layer of more hydrous material. Under the experimental conditions and after 24 hours oxidation, the constructed Eh (oxidation potential) vs pH diagram revealed that CuO was, indeed, the predominate stable product to be expected.
Surface oxidation of chalcocite proceeds at a moderate rate, and it is substantially complete in 24 hours as demonstrated by the experimental data. Within the 24-hour period, interfacial changes occur as manifested by the changes in sign and magnitude of the measured zeta potentials (electrokinetic potentials) which are dependent upon the initial pH used in the oxidation procedure, time of exposure to dissolved oxygen and the interfacial activity of ionic species derived from the mineral surface.
After 24-hour oxidation, constancy of electrokinetic (zeta) potentials with time indicates rather complete surface oxidation, but not necessarily a discontinuation of oxidation. Quite likely, oxidation continues at a slower rate by diffusional processes beneath the surface film.
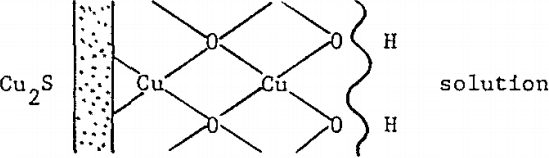
As previously noted, water adsorbed appears as hydroxyl groups in infrared spectra (See spectrograms 2, 3, 4 and 5 wavenumber 1380 cm-¹ Fig. 8). Such surface hydration can be depicted as follows:
With increasing basicity, variability in the IEPS’s are encountered. Contrastingly, in the acid environment, the Cu++ ions predominate in interfacial influence because the sulfur-containing ions are in very limited concentrations. But, in the basic environment, the concentration of sulfur-bearing ionic species is appreciably greater. In other words, the electrical double layer becomes a cationic donor on the basic side of IEPS. The exchange capacity of the sulfur- bearing ionic species varies with pH as it is increased above the IEPS.
At a collector concentration of 0.5 x 10 -4 M of sodium isopropyl xanthate, the recovery decreases sharply within the pH range 3 to 9. In the pH range 5 to 9, oxidized chalcocite responds to the xanthate collector as if it were an oxide. Above pH 9, recovery increases slightly. Precipitation of copper hydroxide is likely which would reduce the concentration of free copper ions; hence, the possibility of precipitating copper xanthate. However, the surface cupric oxide remains practically unaltered.