Using Rock Bolts or Wire Rope to Pillar Strength
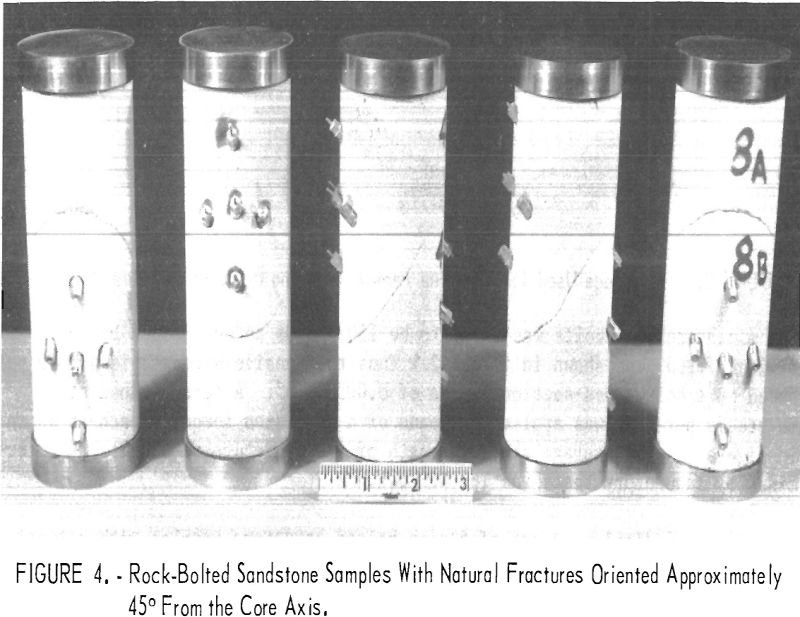
In a room-and-pillar system of mining in which no artificial support is used (linings, sets, props, etc.), the weight of the overlying cover is sustained in part on the abutment and in part by the pillars. The pillars are usually the most important structural support element in a room-and-pillar system and their stability is responsible […]
Coal Free-Swelling Index Testing
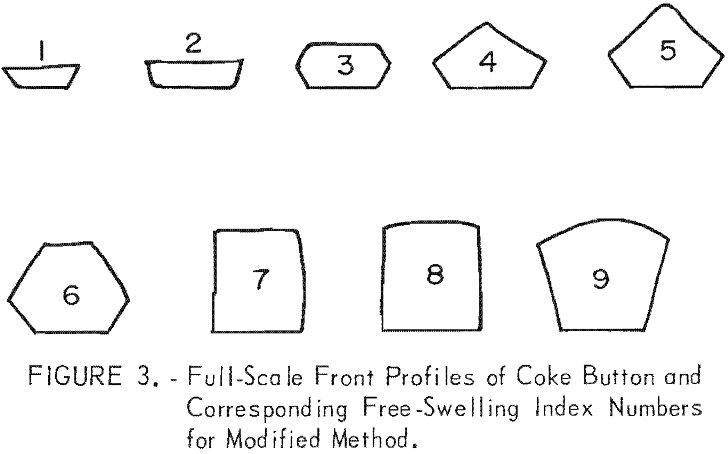
The term “free-swelling” is applied to the behavior of some bituminous coals when heated under specified conditions. Damm defined the swelling of coal as the volume change that takes place when coal is heated. The softened coal can expand, for example due to gases being released on pyrolysis, in the direction away from the heating […]
How to Control Sulfur Oxide Emissions in Smelting

The domestic base-metal-producing industry has been challenged with the task of reducing the amount of sulfur oxides and other pollutants emitted by smelters treating sulfide ores and concentrates. This challenge has been imposed by legislation aimed at reducing air pollution. Standards for both ambient air quality and allowable emissions have been proposed and already are […]
How to Recover Cadmium & Nickel from Scrap Batteries
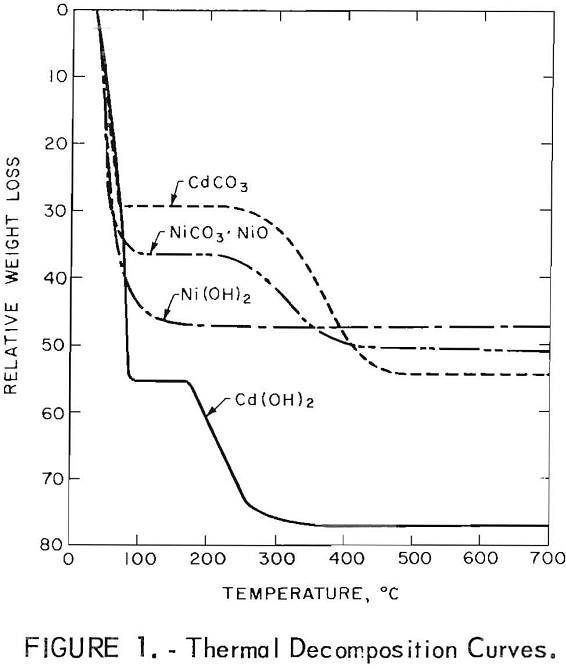
The manufacture and use of nickel-cadmium alkaline storage batteries in the United States began to grow at a rapid pace in the late 1950’s. The batteries have found use in a wide variety of consumer appliances, electronic units, industrial equipment, communication devices, and space applications. The U.S. Department of Defense is the biggest single user […]
Recycling and Disposal of Solid Mining Wastes
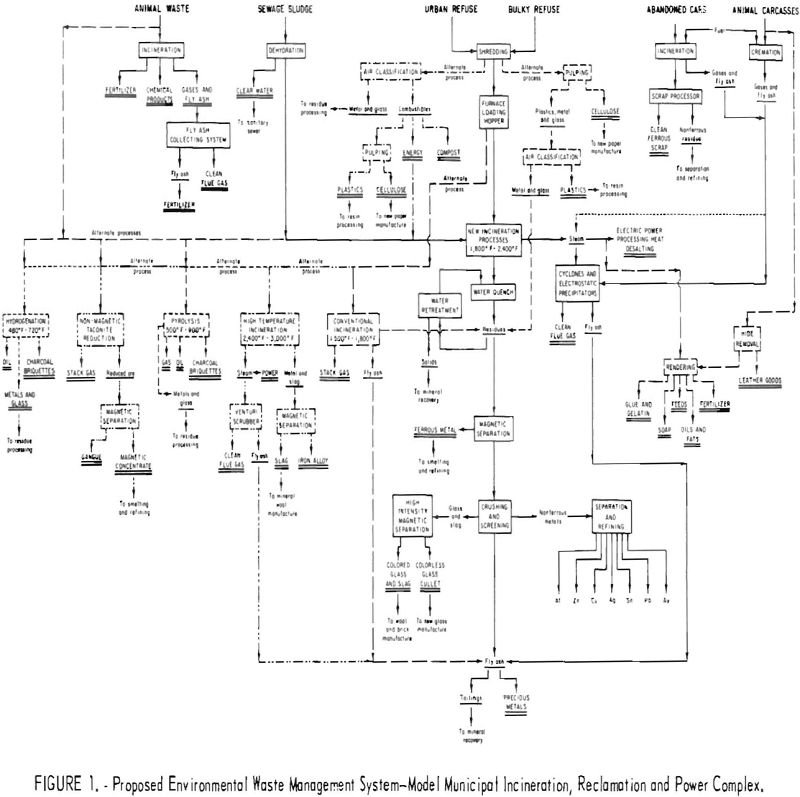
The Interior Department’s Bureau of Mines has always considered the waste products and scrap generated by the mineral and metals industries and the consuming public as potential resources, The Bureau has been active in reclaiming values from byproducts of mineral, metal, and energy processes for over 50 years. This statement is supported by the continuity […]
How to Recover Copper from Slag by Flotation

The bulk of the world’s output of copper is produced by smelting copper sulfide flotation concentrates in reverberatory furnaces, followed by oxidizing the matte to blister copper in a converter. Slags produced in the converter are too high in copper to be sent to the dump and are returned to the reverberatory furnace for recovery […]
Electrowinning Tungsten
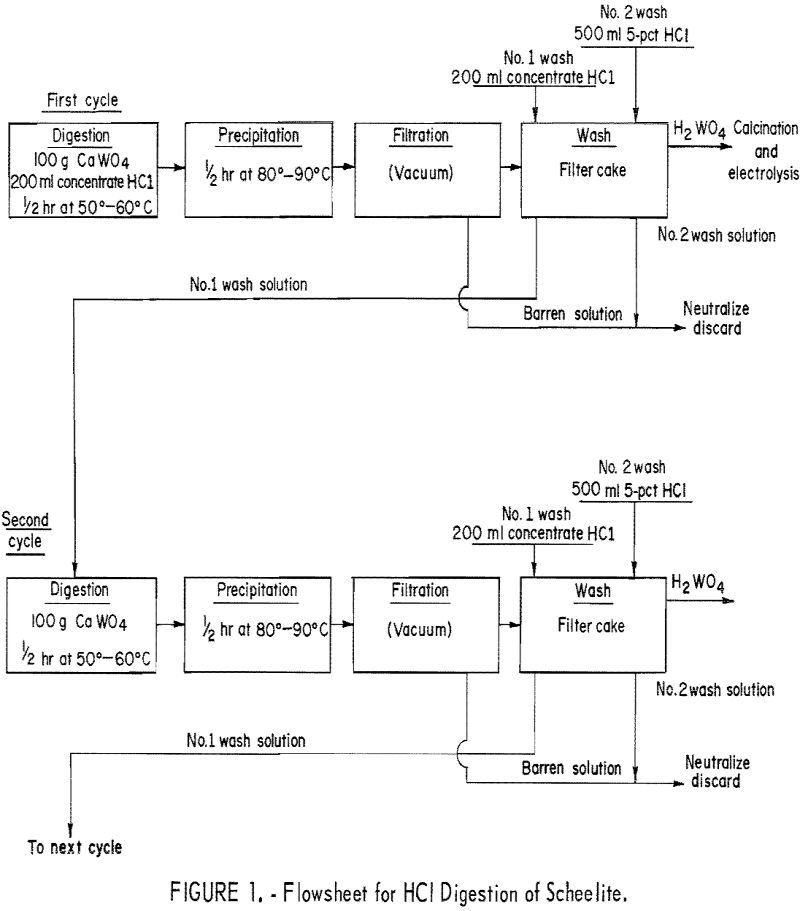
We demonstrated techniques for the electrowinning of tungsten directly from scheelite concentrate. The major problem with direct electrowinning is the accumulation of lime (CaO) in the electrolyte. Scheelite mineral concentrates usually contain 60 to 70 weight-percent WO3 and about 20 weight-percent CaO. The results of the CaO buildup are decreased metal purity, increased electrolyte viscosity, […]
Recover Uranium from Water by Countercurrent Ion Exchange
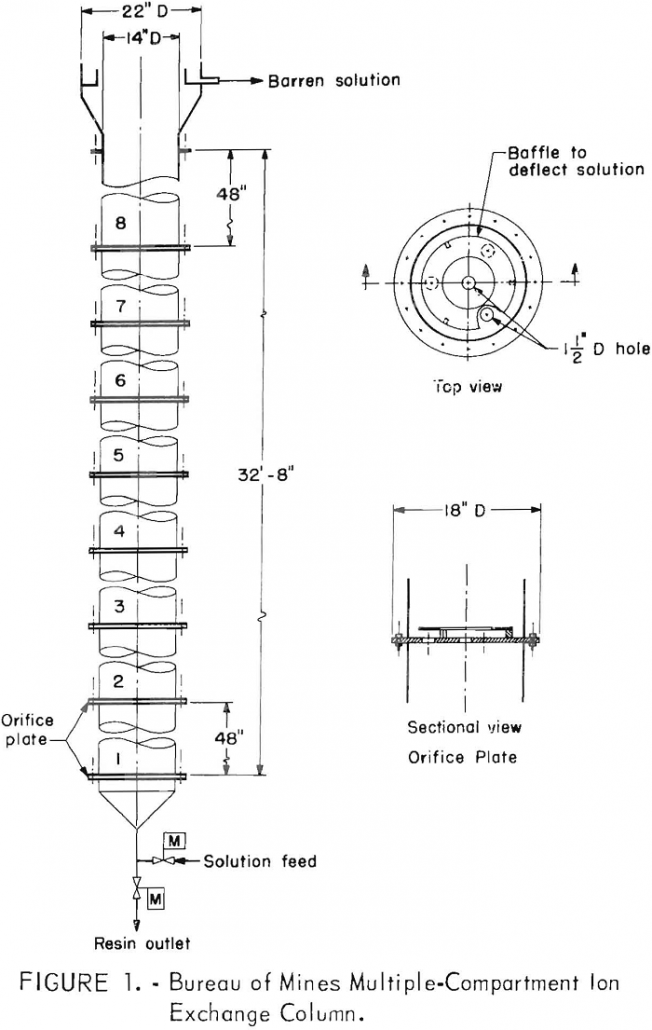
Ion exchange techniques have been used to recover uranium from waters pumped from uranium mines in the Ambrosia Lake district of New Mexico since about 1963. More recently., the natural flow of mine water and the recovery of uranium have been augmented by routinely spraying abandoned areas of the mine with barren solution from the […]
How to Sample Gold Lode Deposits
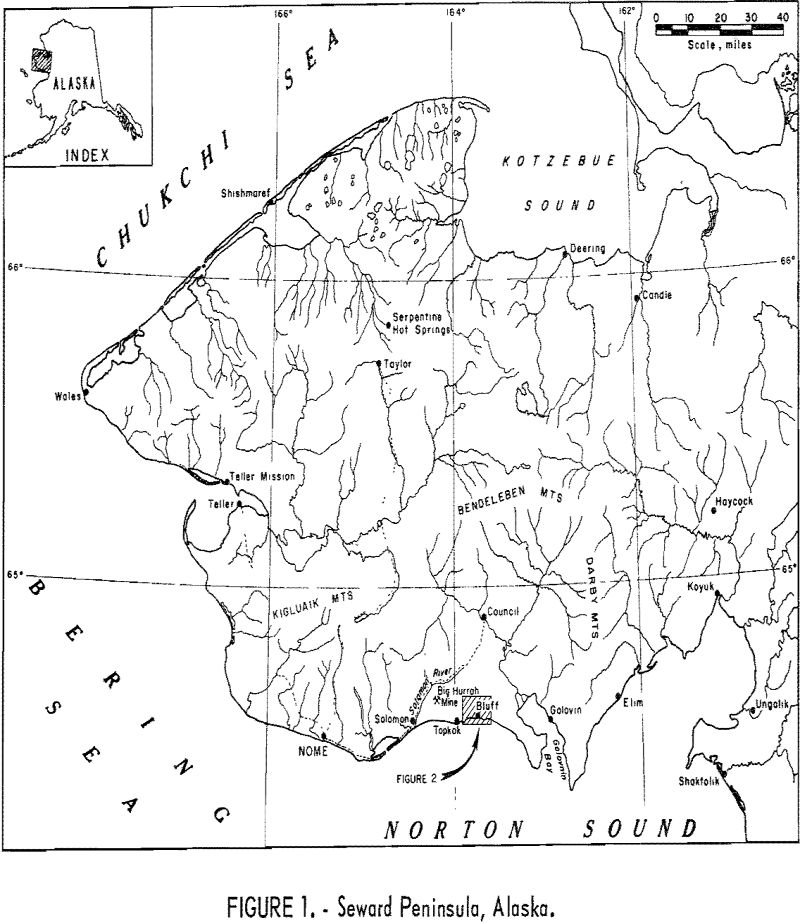
Placer and lode gold deposits near Bluff, Seward Peninsula, Alaska (fig. 1), were investigated during July and August 1966. The primary objective of this Bureau of Mines investigation was to sample a typical gold lode deposit as a basis for estimating the potential economic value of the lode sources of the productive Seward Peninsula gold […]
How to make Bricks from Mill Tailings
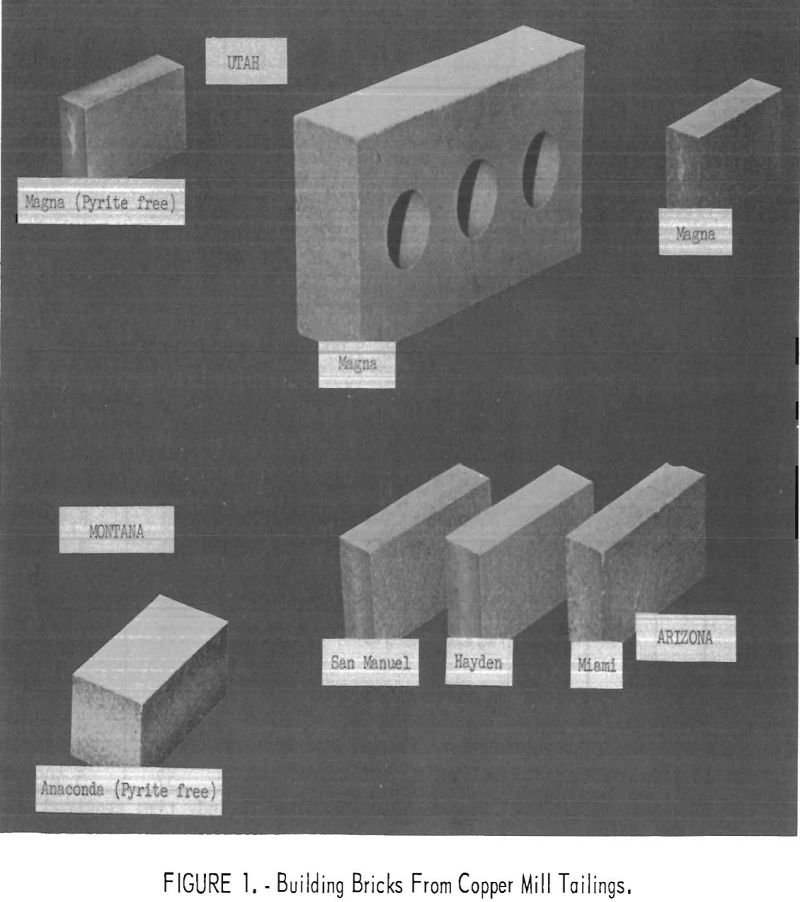
Most of the primary copper produced in the United States is derived from porphyry-type ore deposits located in Arizona, Montana, Nevada, New Mexico, and Utah (Intermountain States). In milling these low-grade ores to produce a smelting-grade concentrate, enormous tonnages of finely ground tailings are discarded. The vast accumulation of these wastes from past operations and […]
