Uranyl peroxide precipitation offers two advantages over other precipitation methods, product purity and product handling. However, since it is a less well known process than ammonia, caustic or Mag-Ox precipitation, it is helpful to demonstrate the advantages of hydrogen peroxide by comparing its performance with the widely known and used ammonia precipitation process.
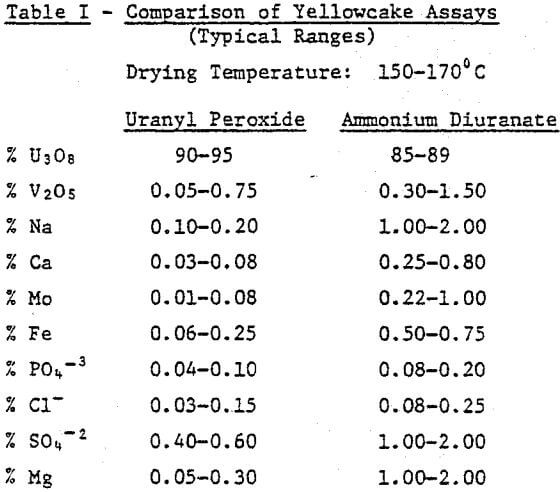
First, product purity is an obvious concern for any uranium producer. The ability to consistently meet yellowcake purity specifications can mean considerable savings for a mill. Table I gives a comparison of assays for uranyl peroxide and ammonium diuranate (ADU). The table is a composite of several comparative tests of uranyl peroxide and ADU, but is typical of what might be expected for specific solutions. As can be seen, hydrogen peroxide consistently produces a purer yellowcake.
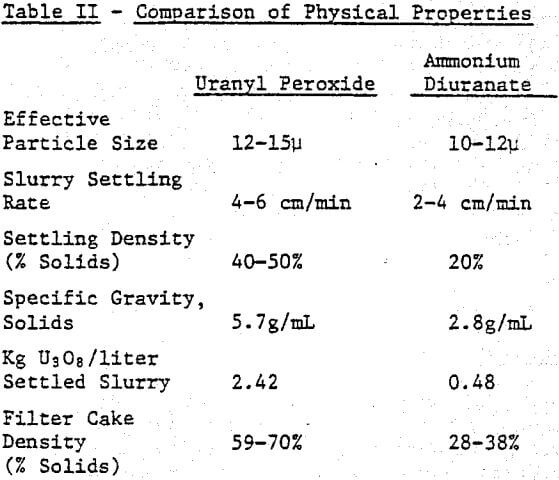
The second consideration in a compartive examination of peroxide and ammonia precipitation processes is the ease of solid/liquid separation. Slurry settling rate, specific gravity of the yellowcake, ease of dewatering, and drying behavior must be evaluated to determine how easily each precipitate can be processed.
Table II compares the physical properties of uranyl peroxide and ADU. Uranyl peroxide is more dense, settles faster, dewaters to a greater extent and has a larger particle size.
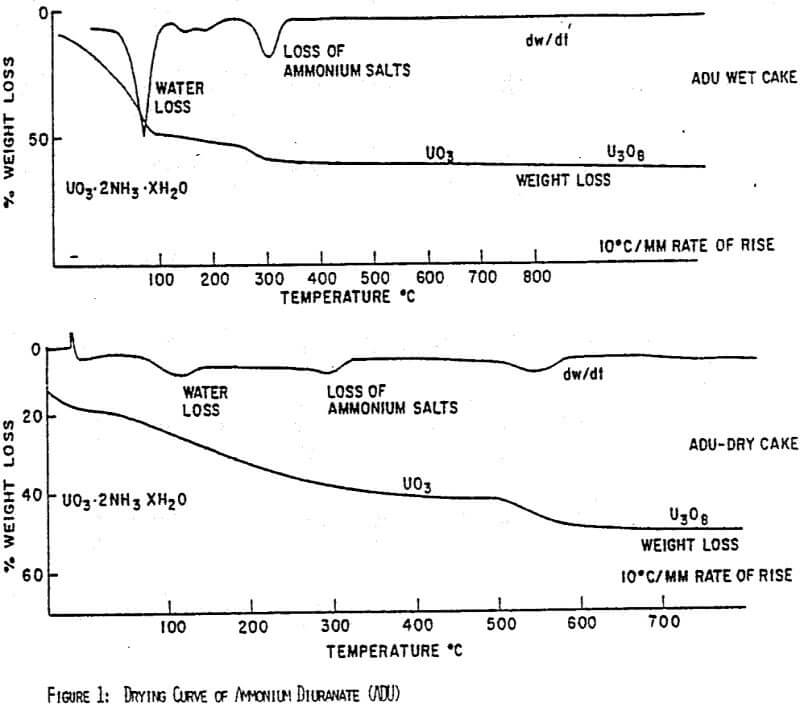
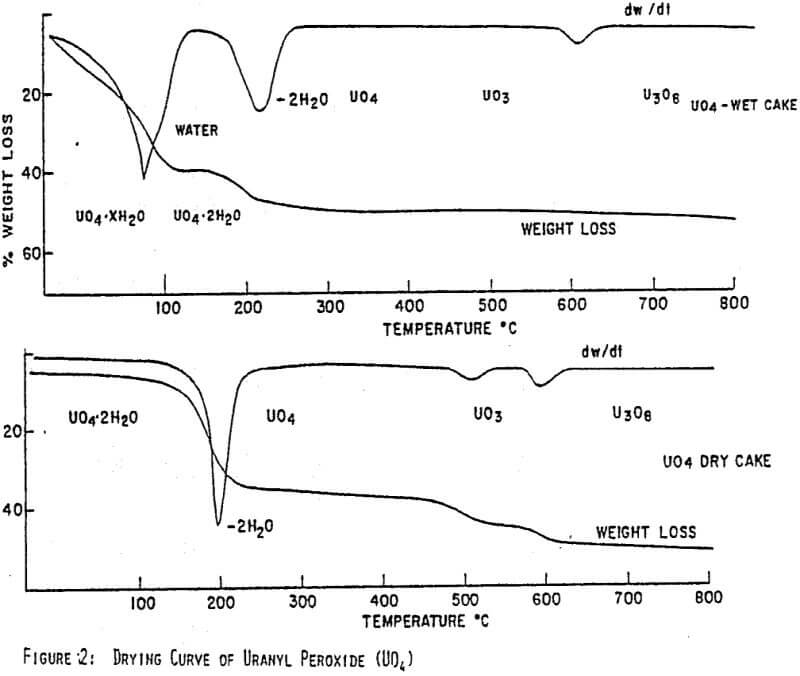
Figures 1 and 2 show the drying curves for uranyl peroxide and ADU. As can be seen, the weight change due to the moisture loss at 100°C is much greater for ADU as can be expected from its lower % solids in a filter cake. As the temperature increases, uranyl peroxide loses its crystalline water at 150-220°C giving UO4. ADU, however, does not lose the ammonium ion that is generally incorporated chemically until the temperature reaches 300-350°C. Consequently, uranyl peroxide can be dried to a high assay product at 150-200°C, whereas, ADU must be heated to at least 300°C. This difference is easily seen in the comparative assays of yellowcakes dried at 150°C; uranyl peroxide will assay -96% U3O8, while ADU will assay only -89%.
Uranyl Peroxide Process Chemistry
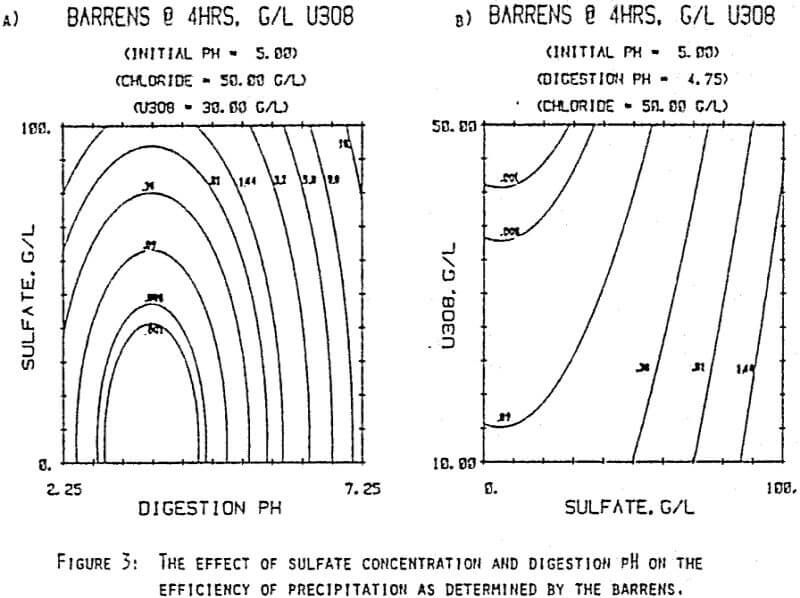
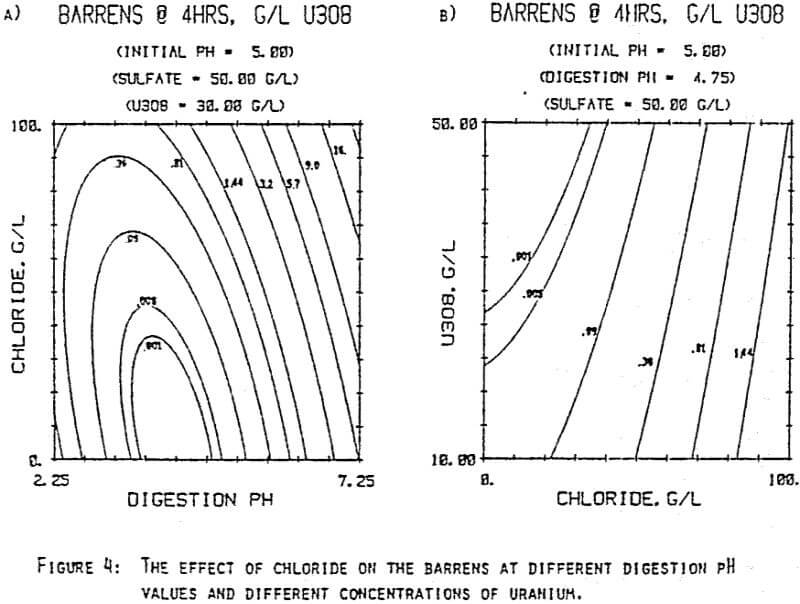
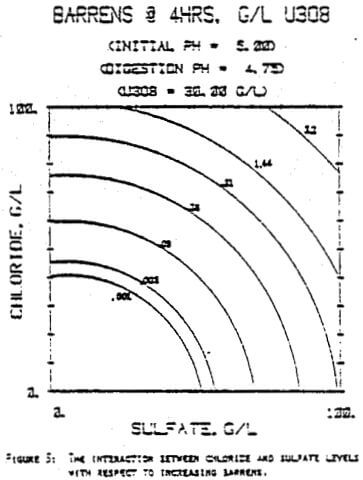
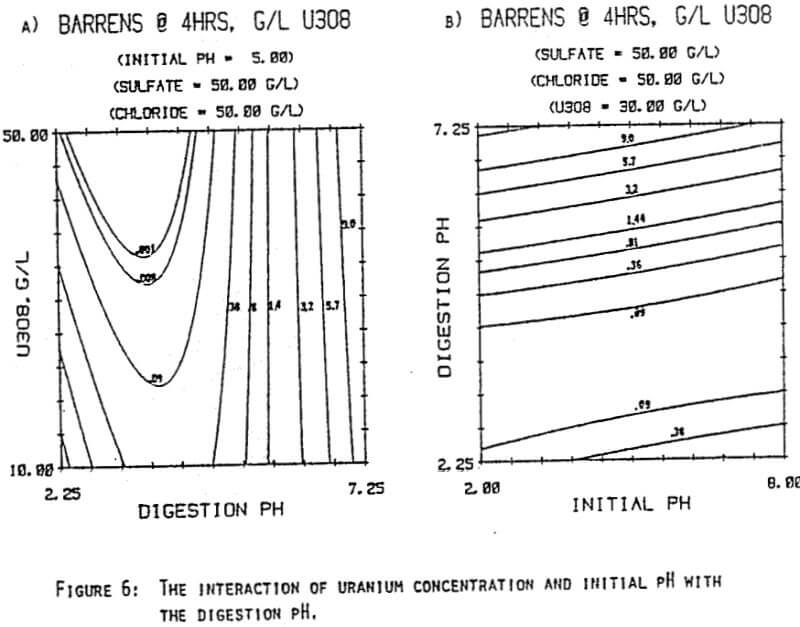
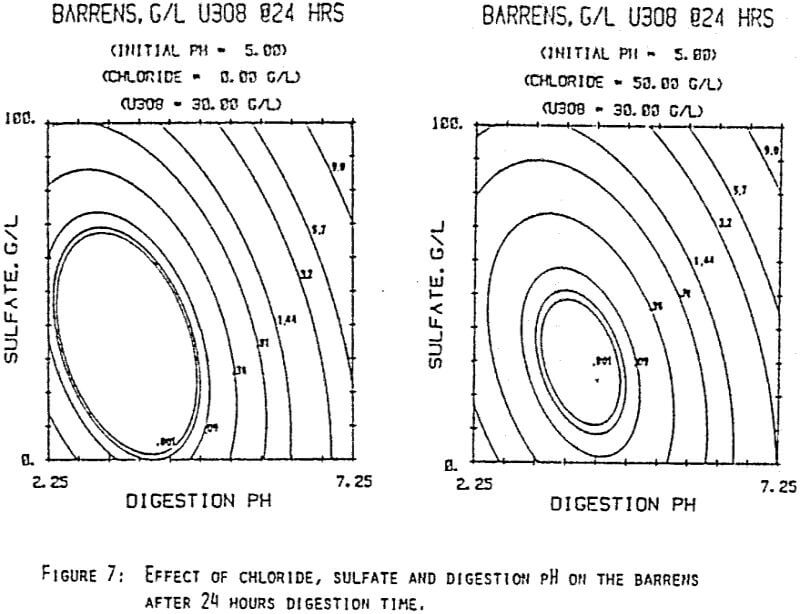
Since uranyl peroxide is demonstrably more easily handled and of higher purity than other yellowcakes, the most important criteria to be evaluated in considering the use of hydrogen peroxide is the ability to attain satisfactory barrens. The precipitation reaction and, hence the barrens, is affected by a number of factors— pH, ratio of peroxide to uranium, and the presence of anions that can complex the uranium. The proper operation of the uranyl peroxide precipitation process necessitates a good understanding of these factors and of their interactions.
In its simplest form, the reaction of hydrogen peroxide with uranium can be written as:
![]()
From this reaction it is apparent that raising the pH or increasing the peroxide added will promote the formation of uranyl peroxide.
Uranium can form complexes with anions such as chloride and sulfate (3):

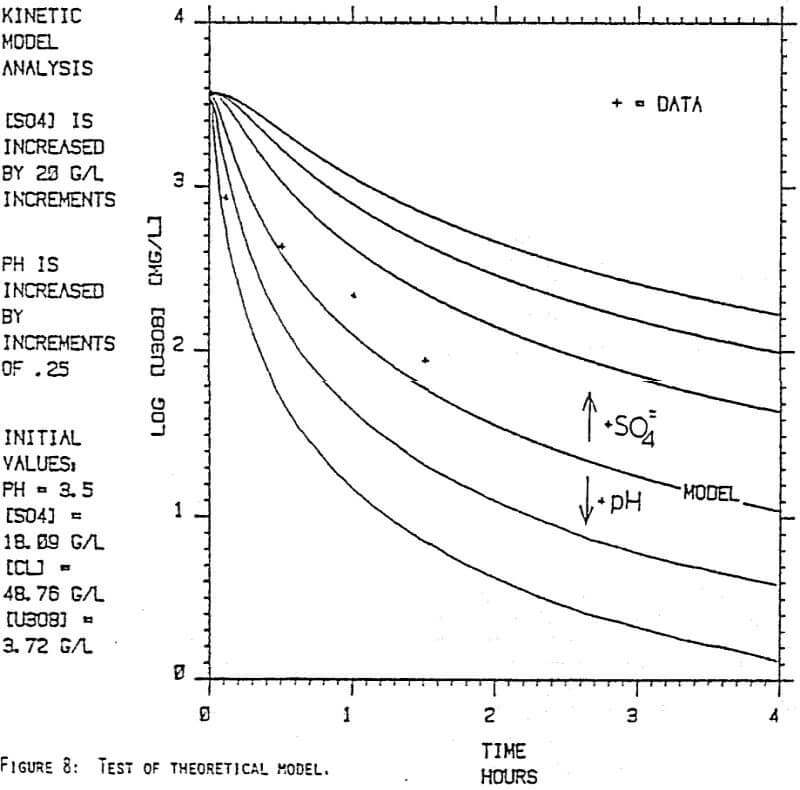
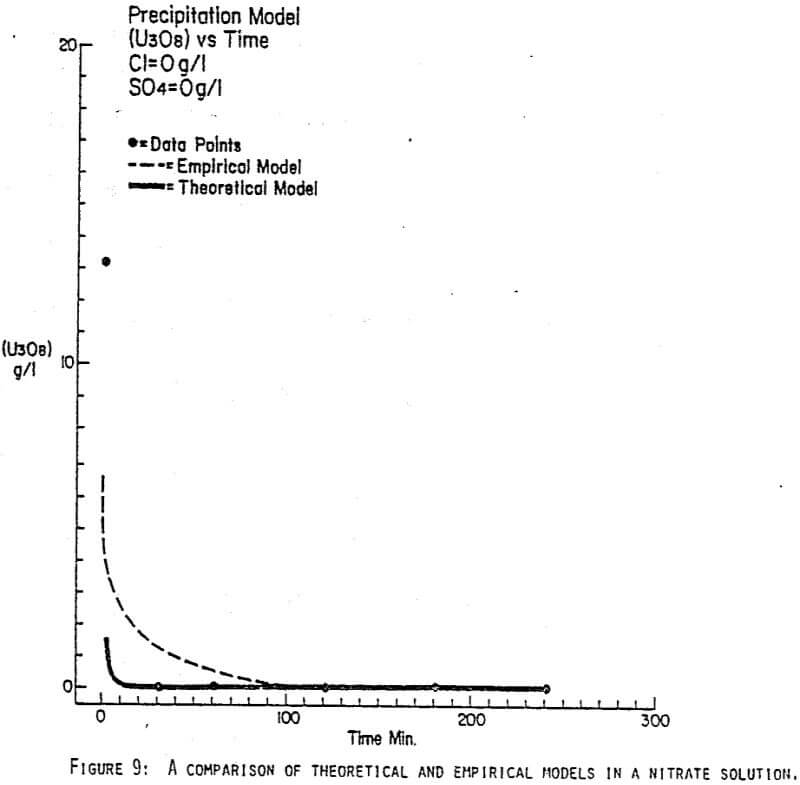
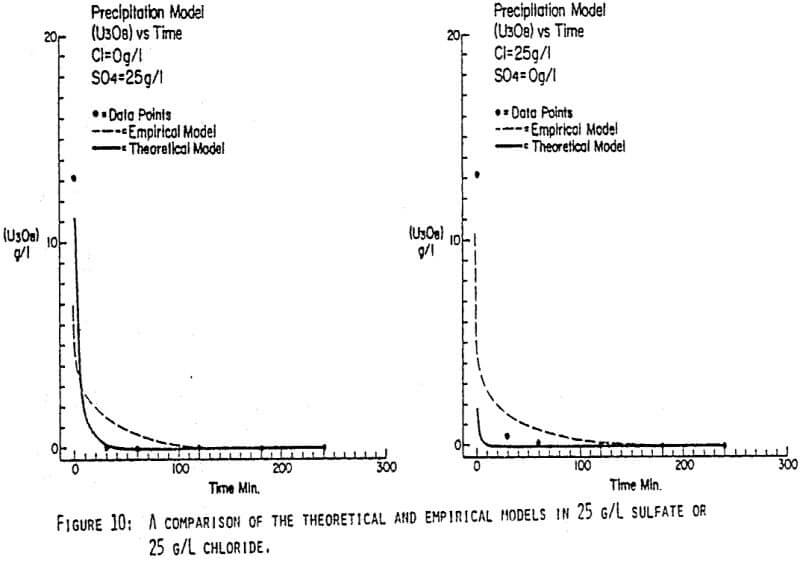
which will inhibit the formation of uranyl peroxide by decreasing the concentration of free uranyl ion. For example, a uranyl nitrate solution can be quantitatively precipitated with a peroxide-to-uranium ratio of .15 at a pH of 2.0; however, if either sulfate or chloride are added, the conditions change dramatically—both the pH and the peroxide must be increased to attain quantitative precipitation.
The procedure used for each experiment was as follows:
A stock solution was doped with chloride and/or sulfate. The pH was adjusted to the design value and .19g H2O2/g U3O8 was added. The pH was readjusted to the digestion pH value and the solution was stirred. Samples were taken at four and twenty-four hours for barrens analysis.
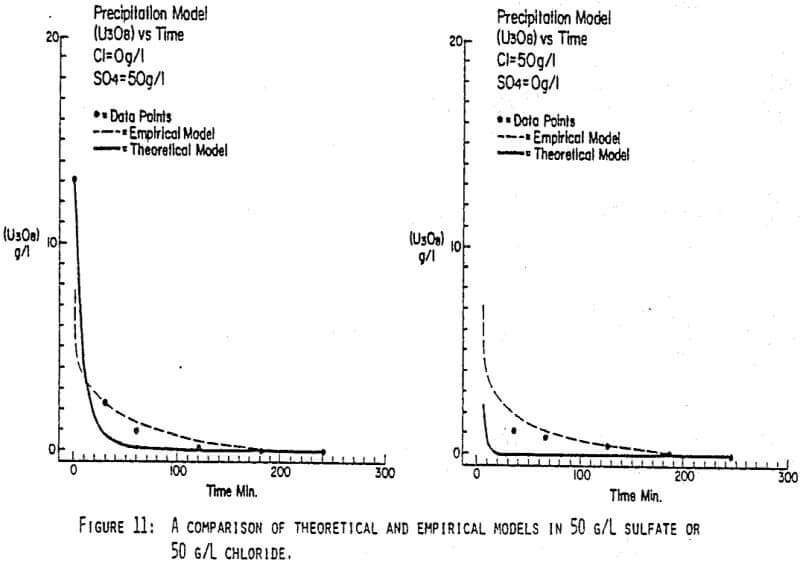
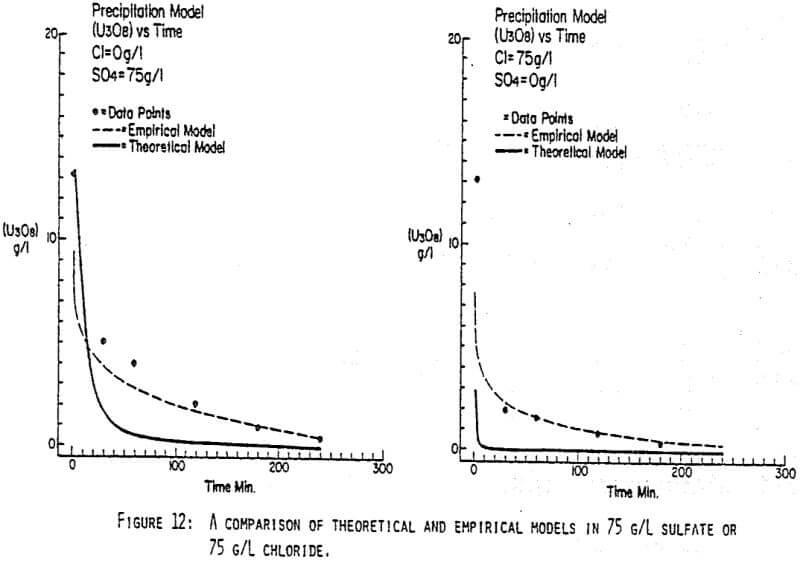
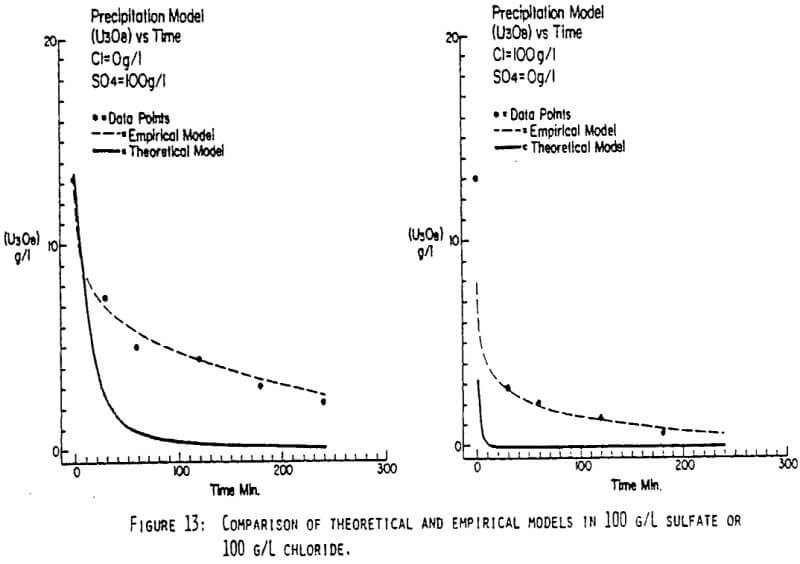
After the experiments were run, the barrens were analyzed to determine the dependence of the barrens on any of the variables.This model is most clearly expressed graphically as contour plots of the barrens.