The rate of evaporation of a volatile metal from a metallic solution containing less volatile metals is determined by its partial pressure and its diffusivity. The concentration of a volatile metal will be less at the surface than in the interior of the metal; it will adjust itself to keep the rate of diffusion and the rate of evaporation in balance. At higher concentrations, the rate of evaporation is determined primarily by the volatility of the volatile metal; at very low concentration the limiting factor is diffusivity.
Distillation, as a metallurgical technique, is most useful for refining volatile metals, or for separating a volatile metal from a mixture of metals. It is much less effective for the complete removal of a volatile metal from other metals. Although cadmium and zinc can be removed from lead and tin, down to about 0.1 atomic percent, further removal is exceedingly slow. For less volatile metals, or metals such as magnesium, which form, intermetallic compounds with lead and tin, the practical limit is about one percent.
Selective distillation has long been one of the useful techniques of the chemical engineer for separating organic compounds. Distillation also offers a way to separate metallic mixtures. It is one of the few techniques whereby metals can be separated from each other without being changed from the metallic state. Most refining procedures involve downgrading of one or more of the constituent metals into a dross, slag, or residue, which has little value or requires further processing to recover the constituent metals in a pure or usable form. In principle it is possible to separate as completely as may be desired, a binary mixture of a volatile and non-volatile metal, and to recover both metals in a relatively pure state.
For several years the Bureau of Mines has been studying vacuum distillation as a process for separation and refining of metals, particularly secondary metals from non-ferrous scrap. By reducing gas pressure within the distillation apparatus, the temperature required for distillation is decreased.
Decreasing the pressure of the residual gas allows more rapid diffusion of the evaporating metal away from the surface, and permits distillation at partial pressures of only a few millimeters.
Distillation is particularly advantageous as a means of refining volatile metals such as zinc, magnesium, or cadmium. However, it also offers a means of removing a small amount of a volatile metal from a less volatile one. Previous work has been reported on distillation of zinc from aluminum-zinc alloy and this report summarizes work on the removal of the more volatile metals, such as cadmium, zinc, and magnesium, from lead and tin.
A similar study was made by Kroll. He was able to volatilize arsenic, lead, and silver quite completely from tin. The lead condensate contained more than 50 percent lead, but the other condensates were predominantly tin. Neither antimony nor bismuth was selectively removed from lead containing less than one percent of the impurity.
Volatility of Metals and Alloys
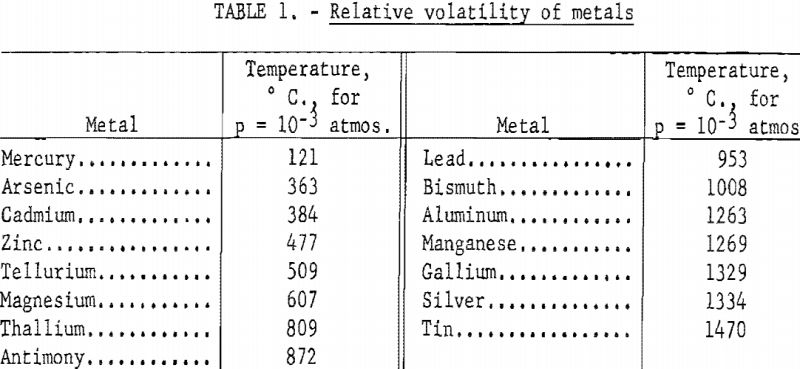
The relative volatility of the common metals is shown in table 1. The metals are listed in order of increasing volatility, as determined by the temperature required to produce a partial pressure of 10-³ atmospheres. The data are taken from vapor pressure data assembled by Kelley.
When metals are mixed together as a molten or solid solution, they no longer have the same relative volatility as in the pure state. The vapor pressure of each metal is proportional to the product of its mole fraction and its vapor pressure in the pure state:
p = γn po……………………………………………………………………(1)
where p = partial pressure for solution
po = vapor pressure in pure state
n = mole fraction
γ = activity coefficient
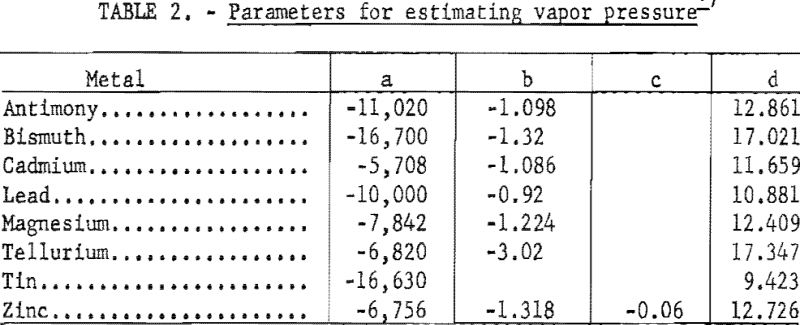
The vapor pressure po increases rapidly with increasing temperature. It can be represented by the following equation:
log p = a/T + b log T + c T + d……………………………………………….(2)
where p = vapor pressure in mm
T = temperature, °K.
a, b, c, & d = parameters characteristic of each metal as shown in table 2.
The limiting values of γ for pairs of metals for which measurements are available are listed in table 3.
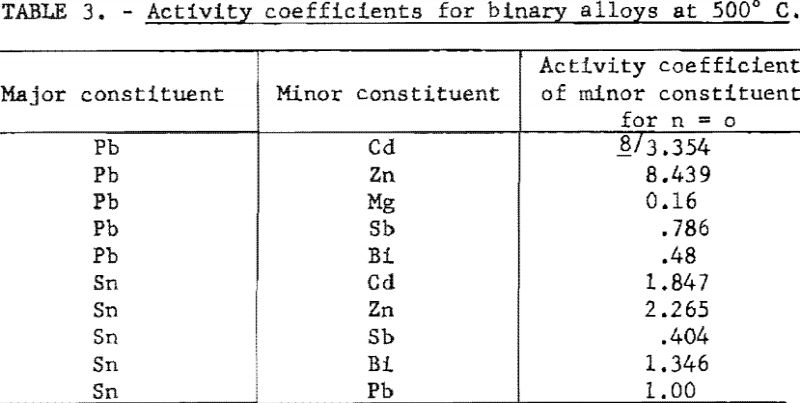
The activity coefficient γ is a coefficient which takes into account departure from Raoult’s Law. The value of γ is a function of the atomic fraction n. It approaches a value of 1 as n approaches 1. For pairs of metals, such lead and tin, that obey Raoult’s Law it has a constant value of 1. However, for most pairs of metals it will have a value greater or smaller than n, according to whether the two metals tend to be immiscible or to form intermetallic compound. For example, the activity coefficient of zinc in lead shows a positive deviation from Raoult’s Law (γ>1) whereas magnesium shows a negative deviation (γ<1).
The rate at which a metal will evaporate is proportional to its vapor pressure. When a metal is evaporating into a vacuum, or when the metal vapor diffuses away from the surface as fast as it evaporates, the rate of evaporation is given by

where w = rate of evaporation in grams per sq. cm. per sec.
P = vapor pressure of liquid in dynes per sq. cm.
M = molecular weight of evaporating metal
T = temperature, °K.
The maximum rate at which a metal will evaporate at any temperature, T is given by

where P is now the partial pressure of metal vapor in millimeters of mercury.
Since the partial pressure of a metal in solution is proportional to its molar concentration, (Equation (1)) the rate at which a metal is removed from the melt by evaporation at constant temperature, is expressed by the differential equation:
-dx/dt = kx……………………………………………………………………(5)
where x = concentration of volatile metals in the melt
k = rate coefficient
t = time
If we assume that the coefficient k is a constant and that xo is the initial value of x at t = o, the solution of (5) is

That is, the rate coefficient can be determined experimentally by determining the rate of change of xo/x over short intervals.
The rate coefficient, k, is constant only when evaporation from the surface is the rate-determining process. For rapid evaporation or when the concentration becomes very low, the rate of evaporation will be determined by the rate of diffusion of the evaporating metal to the surface. Or, in other words, the concentration at the surface will be such as to bring the rate or diffusion and the rate of evaporation into balance.
The interaction of diffusion and evaporation can be shown as follows:
x = concentration of evaporating metal in body of melt
xs = concentration at surface
α = rate coefficient for evaporation
β = rate coefficient for diffusion
The rate of evaporation will be equal to:

The rate constant k in equations (5) and (6) is equal to αβ/α + β. In other words
1/k = 1/α + 1/β………………………………………………………………(10)
or the reciprocal of the overall rate coefficient is equal to the sum of the reciprocals of the evaporation and diffusion coefficients. This means that as α becomes very large k becomes very nearly equal to β; and as β becomes very large, k is very nearly equal to α.
The experiments on volatilization of metals from lead and tin were made to determine the effect of concentration on the rate of evaporation, and the effect of concentration and rate on the composition of the vapor, as the concentration of the more volatile metal becomes very small.
Experimental Procedure
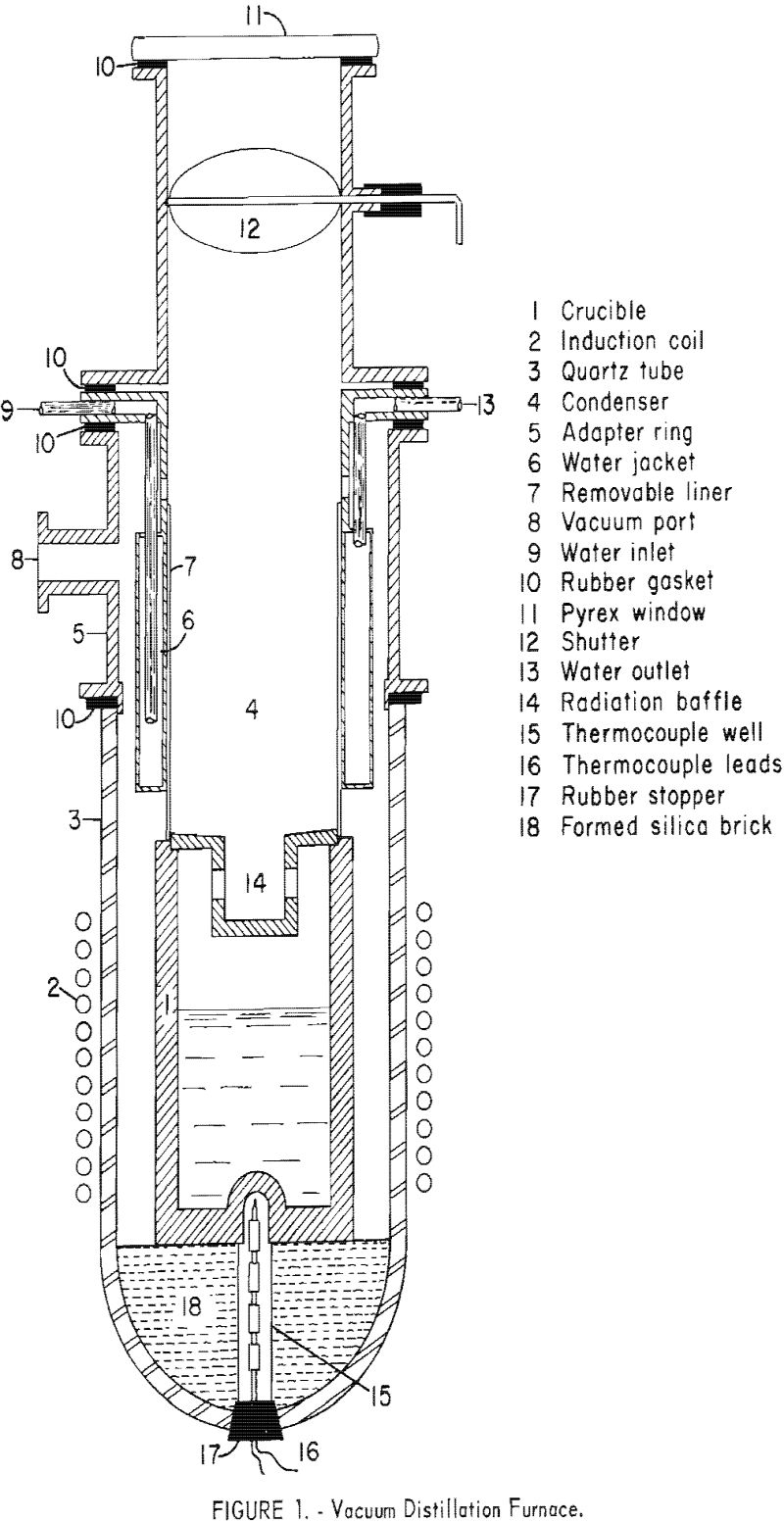
The distillation furnace is shown in figure 1. The crucible and the condenser are enclosed in a large quartz tube, closed at the bottom and open at the top. The condenser is directly above the crucible. A flanged adaptor ring, fitted to the top of the quartz tube, supports the condenser section. The condenser is a water-jacketed brass cylinder, provided with a removable liner, to facilitate stripping condensate from the condenser.
The furnace is evacuated through a 2-inch line, to a mechanical vacuum pump. Rubber gaskets between the connecting flanges insure a vacuum-tight system. The interior of the furnace can be observed through the window above the condenser. A movable shutter is provided to prevent fogging of the window during stand-by periods. A radiation baffle can be placed between the crucible and condenser if necessary to prevent refluxing.
Alloys for distillation were melted, blended, and cast into slugs weighing about 1000 grams each, which fit loosely in the distillation crucible. Samples of the molten charge, taken just before casting each slug, were chill-cast to prevent segregation and prepared for chemical analyses, by making saw cuts through the sample at right angles to each other.
After the charge was placed in the furnace crucible, the system was evacuated to a pressure of 10 to 20 microns, and the temperature was increased slowly to prevent sputtering, as the absorbed gas was released during melting. When the temperature reached a predetermined level, it was held constant during the remainder of the test. For tests in which oxidation might occur, the furnace was cooled under vacuum and filled with helium, before it was opened to the atmosphere. The condensate was stripped by removing and unrolling the flexible condenser sleeve. Samples of the condensate were obtained by collecting the hacksaw chips from several cuts through the condensate crown. A dip sample was taken of the residual metal, in the crucible.
The lead used in these experiments was more than 99.8 percent pure. The principal impurities were copper and bismuth. The tin was about 99.7 percent pure; the impurities were chiefly lead and arsenic.
Experiments on Comparative Behavior of Volatile Metals
Two series of tests were made, to compare the behavior of various metals dissolved in molten lead and tin. The volatile metals were zinc, cadmium, magnesium, antimony, bismuth, and tellurium; lead was included as a volatile metal in the tin series. The pressure was usually about 10 microns at the beginning of the test and rose to about 50 microns at the end. The distillation time was 60 minutes. Both the charge and residual metal were analyzed for the volatile constituent. The condensate was usually analyzed only for lead or tin, unless the proportion of the less volatile metal was unusually large. Only a small amount of condensate was collected, all of which was included in the sample for analysis, to avoid errors in sampling.
The results of these tests are summarized in table 4. The rate of evaporation is determined from the initial and final analyses of the volatile metal. The rate coefficient shown in the final column is given in reciprocal hours. The significance of k can be interpreted as follows: The time required to decrease the concentration of the volatile metal to one half of its original value is equal to
0.693/k
For example, these data indicate that the half-time for cadmium in tin at 600° C. is only about 25 minutes, as compared with 41 hours for antimony in tin at 1000° C. Cadmium, zinc, magnesium, and tellurium can be readily distilled from both lead and tin, down to a fraction of one percent at temperatures of 1000° C. or less. It is not practicable to remove antimony from lead by distillation. It is doubtful whether it would be practicable to remove antimony or less volatile metals from tin by distillation.
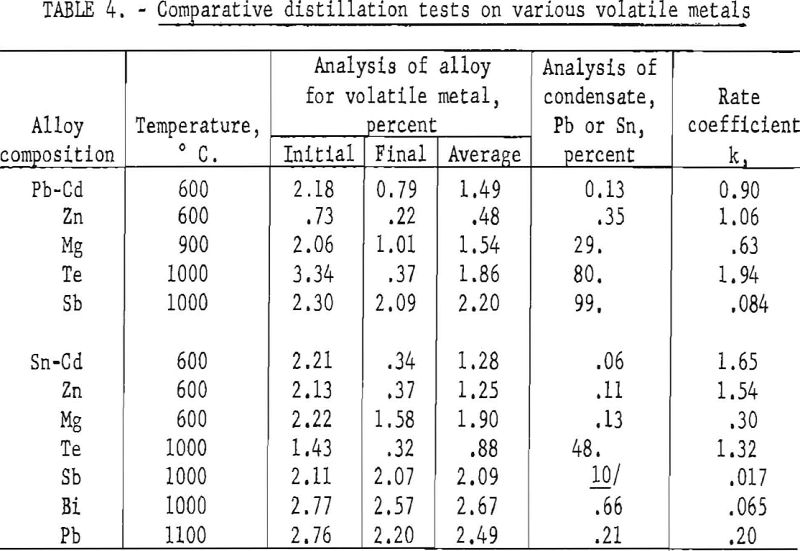
There was an appreciable amount of the less volatile metal in all condensates, except that from the Sn-Sb alloy, particularly in the condensates containing magnesium and tellurium. The condensate from the Pb-Sb alloy was nearly all lead.
The analyses of the condensates from the melts containing tellurium indicate that tellurium is probably volatilized as the intermetallic compound rather than pure metal vapor. This is consistent with the melting point diagrams, which indicate stable β phases having melting points much higher than either constituent. The β phase for the tin alloy has a composition SnTe which corresponds to 48 percent Sn, or the observed composition of the condensate from the tin alloy. The composition of the lead alloy has a fairly wide range, centering around equimolar proportions. The vapor from this alloy is probably a mixture of lead and β phase.
The amounts of the non-volatile metal observed in the condensate are, in most cases, greater than would be predicted from the composition of the alloy and the relative volatility of its constituents. From equations (1) and (3) it is evident that the relative rates of evaporation of the more volatile and less volatile constituents is equal to,

Expressing n in terms of weight percent, this ratio becomes

The ratio of the volatile to the non-volatile constituent in the vapor from a binary alloy can be calculated from equation (12), where x represents the concentration in weight percent of the volatile metal at the surface. Using the average analyses given in table 3 as the value of x, the calculated ratio of cadmium to lead in the vapor is 10,800, as compared with 770 as observed from analysis of the condensate. This would indicate that the concentration of cadmium at the surface is only one fourteenth of that in the interior of the melt. This means, according to equation (8), that the value of α, the rate coefficient for evaporation, is 13 times that of β, the rate coefficient for diffusion.
These ratios are given merely to provide a specific illustration of the effect of diffusion on evaporation. Actually the precision that may have been implied is unwarranted. Similar calculations for other lead alloys indicate that the concentration of the volatile metal in the interior is 10 to 30 times that at the surface. The vapor pressure of tin is not known with sufficient accuracy to warrant similar calculations for the tin alloys.
Distillation of Cadmium from Lead and Tin
Further experiments were made on distilling of cadmium from lead and tin in which the rate was determined for successive intervals of distillation, and at several temperatures. The tests on Pb-Cd alloys were broken into two 60 minute intervals and were run at 500, 600, and 800° C. The tests on Sn-Cd alloys were broken into four intervals and were run at 450, 500, and 600° C. The cumulative condensate for each test was analyzed for lead or tin. The results are given in table 5.
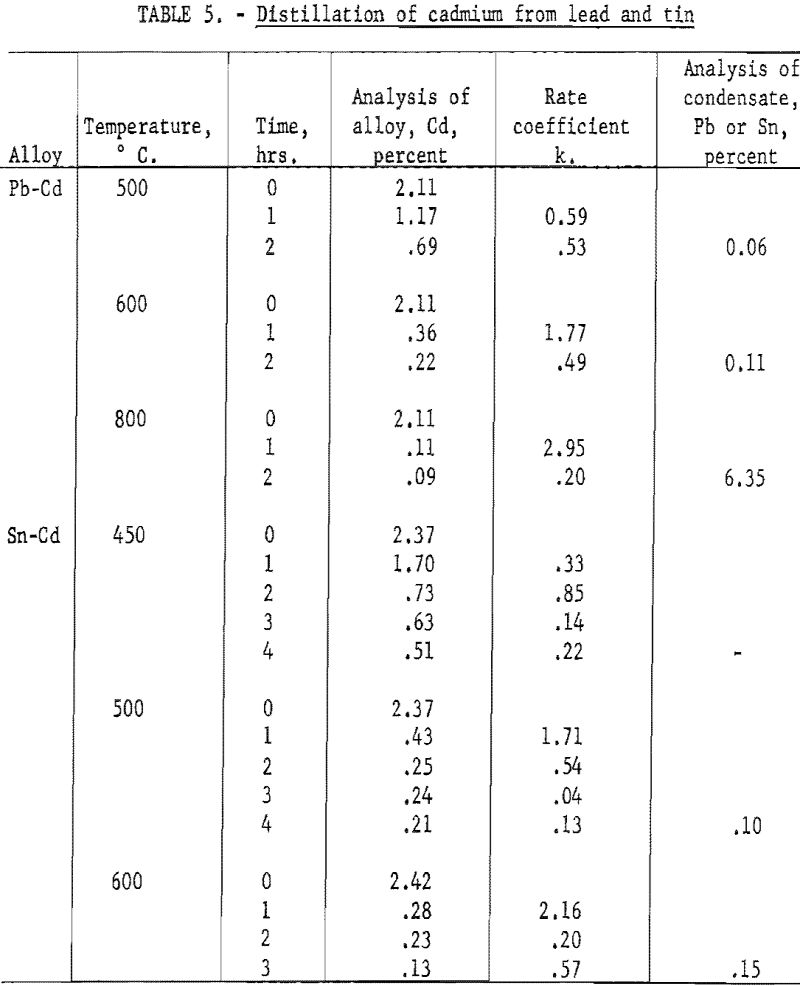
These data show, as might be expected, that the rate coefficient decreases markedly as the volatile metal is depleted. Furthermore, the rate of evaporation does not rise proportionately to the vapor pressure as the temperature is raised. As the temperature increases from 500 to 600 and then 800° C., the vapor pressure of cadmium increases by a factor of 6 and 80 respectively. However, the rate coefficient shows no such increase.
The ratio of cadmium to lead in the vapor for the test run at 800° C., as indicated by the analysis of the condensate, indicates that the average concentration of cadmium at the surface was only 0.017 percent, for a melt varying in composition from 2.11 to 0.09 percent.
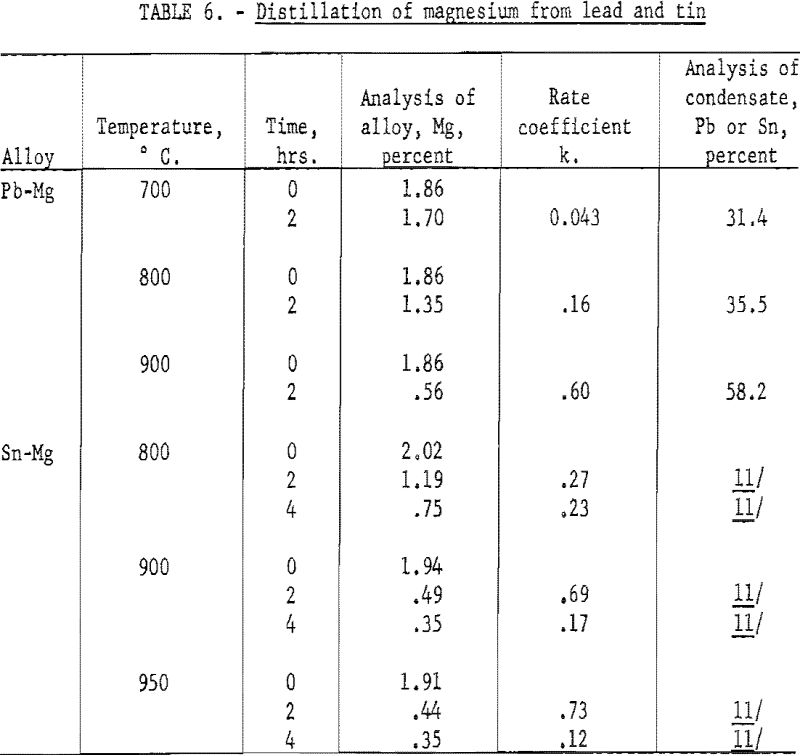
Distillation of Magnesium from Lead and Tin
The experiment on distillation of cadmium from lead and tin is representative of metals that show no tendency to combine, or whose solutions show a positive deviation from Raoult’s Law. Further experiments were made on magnesium in lead, to observe the behavior of a pair of metals that show strong negative deviation. Results are shown in table 6.
The lead content of the condensate indicates that the magnesium content at the surface is in the range of 0.5 to 1.0 percent, as compared with two percent in the interior. Therefore, the decrease in concentration at the surface is not of the same magnitude as in the distillation of cadmium. This is partly explained by the lower atomic weight of magnesium. A two percent alloy of magnesium in lead is nearly 15, atomic percent, as compared with 3.6 atomic percent for cadmium. Thus, from the standpoint of diffusion, the magnesium has a higher concentration and will permit a higher concentration gradient. Therefore, the evaporation is relatively less inhibited by diffusion.
Distillation of Lead from Tin
A final series of tests was made in which lead was distilled from tin at a temperature 1,200° C. These tests were representative of a metallic solution that obeys Raoult’s Law; evaporation was carried out at the highest temperature that the apparatus would permit. The results are shown in table 7.
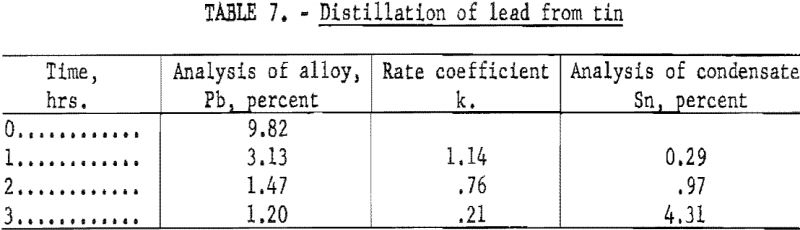
The analyses of the condensate indicated that the average concentration of lead at the surface was 0.40, 0.12, and 0.026 percent for the three successive distillation periods, respectively.
