Water has received little attention as a variable in flotation, even though it is a known fact that water hardness often has an adverse effect on purity and recovery, particularly on the flotation of non-metallic minerals. Because of this fact, process water used in some flotation plants is treated to reduce hardness to an acceptable level. In the laboratory, distilled water is usually used as the flotation medium in order to standardize conditions, whereas in flotation plants locally available water is used.
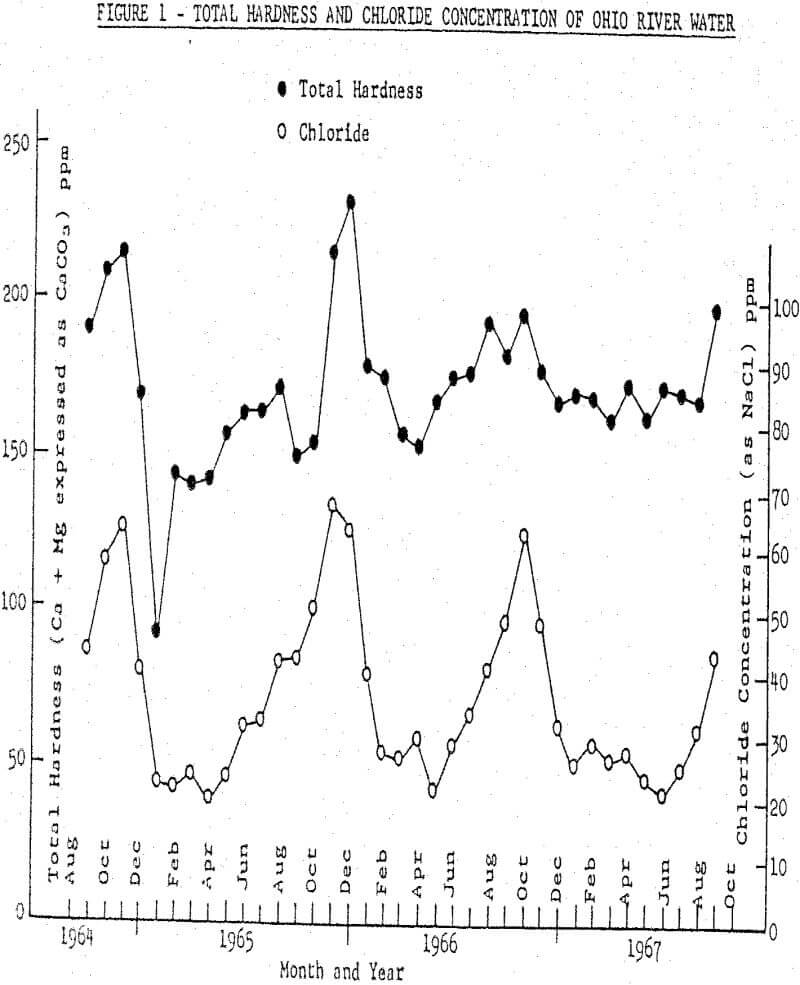
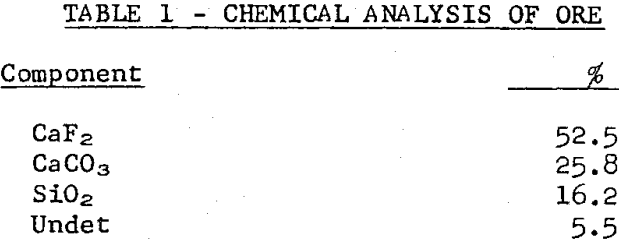
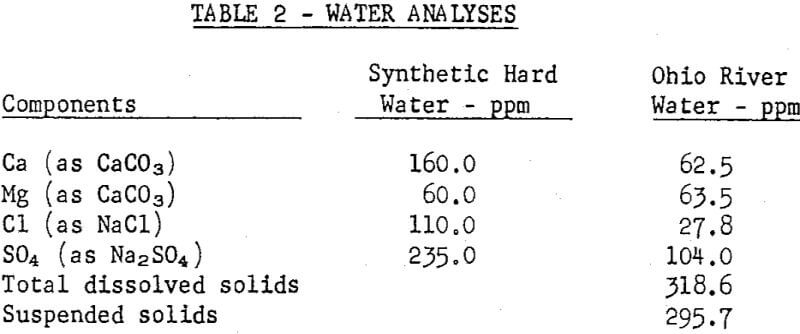
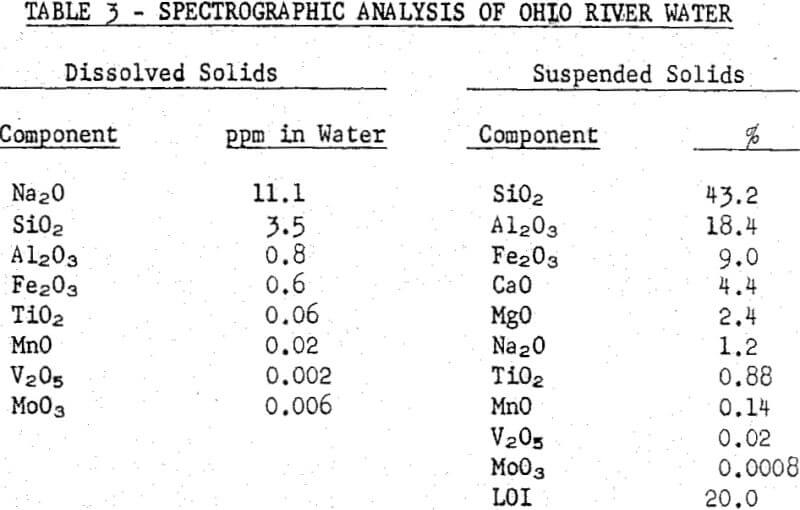
Laboratory flotation tests were run on a sample of the vein-type of ore used at Rosiclare Works in distilled water to which the commonly determined hardness components were added singly, in synthetic hard water and in a sample of Ohio River water. Tests also were run to determine means for counteracting the adverse effects of hard water. All flotation tests were run with pulps at pH 10 maintained with soda ash and at a temperature of 71°C in the rougher float, the conditions used at Rosiclare Works.
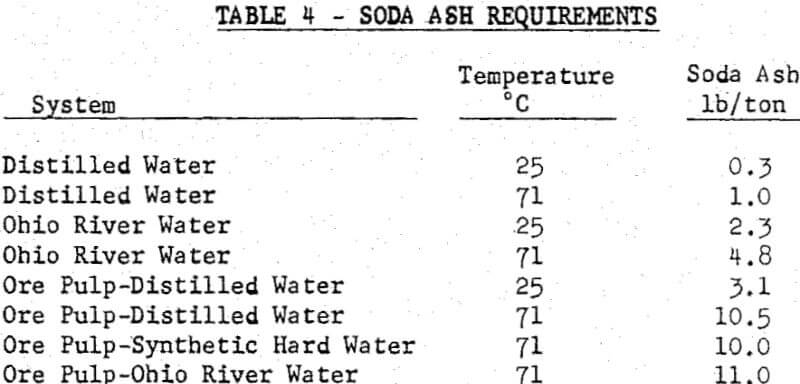
The following conclusions were drawn from this work. More soda ash was required to maintain pH in Ohio River water without ore than in distilled water without ore, the amount increasing with increasing temperature. The amount of soda ash required to maintain pH in a ground ore pulp prepared in distilled water, synthetic hard water or Ohio River water was the same and was approximately 10 times that required for distilled water without ore. There is evidence for the reaction of fluorspar with soda ash at elevated pulp temperatures. Calcium alone had an adverse effect on flotation in small concentrations only and had no effect at large concentrations, while magnesium alone had an adverse effect at all concentrations tested. Chloride and sulfate had no effect, while fluorine had an adverse effect in high concentrations.
The amounts of soda ash required to maintain a pH of 10 in distilled water and Ohio River water alone and in ground ore pulps. The soda ash requirements are expressed in terms of lb/ton of ore for a pulp containing 26% solids so that direct comparisons can be made. For the waters alone, more soda ash is required for the higher temperature, a fact related to the change in hydrolysis constants with temperature.
The experimental data show the following.
- Sodium chloride appeared to have no effect on flotation up to a concentration of at least 100 ppm, with a possible slight adverse effect on grade in going to 200 ppm.
- Sodium sulfate had no effect on flotation in the concentrations used.
- Calcium at a concentration of 42.7 ppm (as CaCO3) had an adverse effect on both grade and recovery, but above this concentration had no effect.
- Magnesium had a small adverse effect on recovery at all concentrations used and caused an increased CaCO3 content of the concentrates with increasing magnesium concentration.