Table of Contents
The study of the condensation of zinc from its vapor was undertaken to shed light on certain problems encountered in large-scale electric zinc-furnace work recently conducted. It is a matter of common knowledge that one of the disadvantages of the arc type, electric zinc furnace, is the production of a large amount of blue powder and proportionately little liquid spelter, for, as stated by Stansfield, “A notable defect in the electric smelting of zinc ores is the difficulty experienced in obtaining the distilled zinc in the liquid state; when zinc ores are smelted electrically, very little liquid metal is commonly obtained, nearly all of the zinc being in the state of powder.”
This difficulty was not encountered in the electric furnace used, the main difficulty being the destruction of the fire-brick lining of the large condenser, in certain parts, and the formation of some oxide and dross coatings on the condensing surfaces. The reason for these difficulties could readily be explained by the infiltration of air whenever the condenser was changed from one retort base to the other, the zinc absorbed in the fire-brick forming oxide and causing rupture by its expanding volume. Two facts, however, tended to disprove this theory for the rupture of the fire-brick lining: first, the large distilling retort, lined with firebrick, which was shifted from charge to charge on alternate bases at temperatures between 1200° and 1300° C., fully exposed to the air, showed no signs of disintegration whatever; second, marked disintegration in the condenser occurred only in places that were at temperatures ranging between, 550° and 450° C. The fire-brick used in the condensers was a Mexico, Mo., brick of excellent grade, but containing numerous “ iron spots.” An examination of ruined brick and tile showed that these iron spots had been filled with fine sooty carbon, and that rupture was due to the increase of volume due to this deposition. This is a comparatively rare, but well-known phenomenon in the iron blast¬furnace, and is clearly described by Frank Firmstone.
The action is due to the breaking up of carbon monoxide into carbon dioxide and carbon at the temperature, at which this reaction is most active (600-450° C.), in the presence of the catalytic agent iron (reduced from the iron oxide by the CO).
This discovery introduced a new problem into the condensation of zinc vapor in large condensers: Is enough carbon dioxide formed by the breaking down of carbon monoxide to interfere seriously with condensation? While practically no blue powder had been formed in the experiments, it was thought that the dross and rock oxide might be due to the carbon dioxide; although the infiltration of air was sufficient to explain their presence, nevertheless the CO2 might be responsible for it in the absence of oxygen. In the circumstances, analyses of gas taken at the exit of the condenser were considered of no particular value, since, if the CO2 acted on the metallic zinc vapor to reoxidize it, more CO would be formed, and the fact that there had been a decomposition of CO could not be detected. That the decomposition of CO has an effect on the condensation of zinc has been suggested by Stansfield.
A search of the literature bearing on condensation revealed practically no facts, nearly all of it being speculative. Data on the composition of gases arising from the distillation of zinc ore are practically limited to those given by Ingalls, which are also stated by R. G. Max Liebig. No record could be found for the equilibrium conditions of the reaction
Zn + CO2 ⇔ ZnO + CO
except general statements, which are referred to in Part III.
Distillation Products from Reduction of Zinc Ore
Method of Investigation
Experiments were conducted under rigorous conditions to determine the composition of the gas and the condition of the condensed zinc from the distillation of zinc ore. For this purpose porcelain and quartz tubes were used, having been demonstrated to be tight and refractory enough for the temperatures employed. Zinc at high temperatures has great affinity for oxygen, removing traces of oxygen from gases, just as copper does. For this reason it was necessary to insure that oxygen
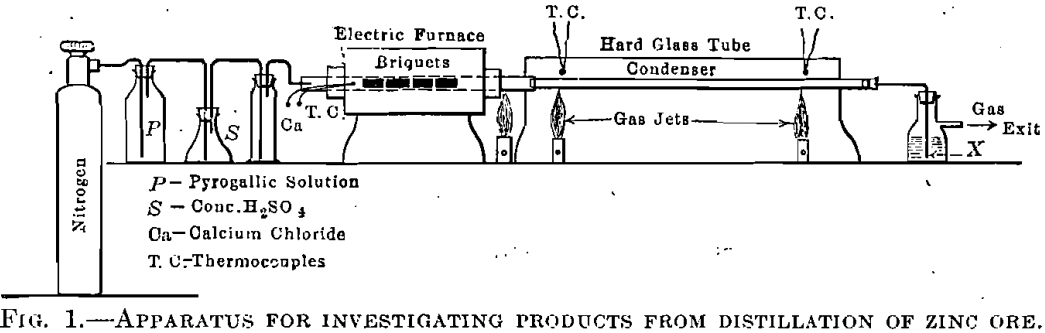
was excluded from the system if the relation between the condition of the zinc and the normal gas composition was to be judged. The arrangement of the experimental apparatus is shown in Fig. 1 and 2. The retort part of the tube was heated in a horizontal, granular-resistance, tube furnace similar in design to the vertical tube furnace used by the author in previous work. The condenser part of the tube was heated, when desired, by a gas-fired combustion furnace. The charge for distillation was in the form of briquets, made of 100 parts Missouri calamine ore, 80 parts of crushed coke, and 20 parts of hard coal-tar pitch. These briquets were approximately 1 in. (25.4 mm.) in diameter and 1¾ in. (44.4 mm.) long and were quartered longitudinally to fit inside the tube. They had previously been baked at a temperature of about 500° C., to drive out the most readily volatile constituents, and contained 18.5 per cent. zinc. The ore contained 39.5 per cent, zinc, 35 per cent, insoluble matter, considerable water, and some carbon dioxide. In composition the briquets were the same as those used in the large electric furnace at East St. Louis, III., and do not differ from the charge of the common zinc retort. The gases from the distillation of this material should resemble zinc-retort gas after the charge has been thoroughly dried and heated to about 500° C.
Oxygen was excluded by the following procedure: the system was thoroughly tested to insure tightness at the temperature used, the quartered briquets were then introduced into the tube, the system closed, and commercial nitrogen (purified by passage through water, pyrogallic solution, cone, sulphuric acid, and calcium chloride) was passed till all
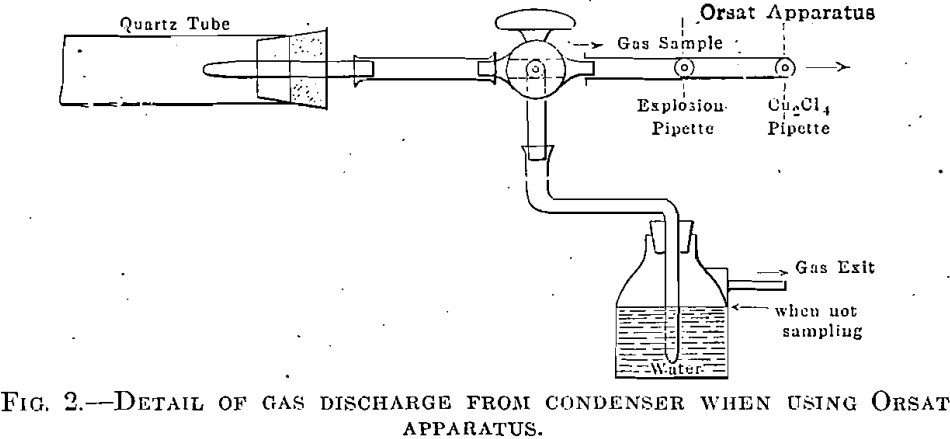
air was considered displaced (usually about 5000 c.c.). The furnace was then heated, and, when the gas flow from the distillation was free and it was thought that the nitrogen was displaced, gas samples were taken and analyzed. The sample was taken by inserting the exit tube X, Fig. 1, which was drawn to a small opening, directly into the water- filled rubber connection of the gas burette. Gas analysis was made with the gas burette and Hempel pipettes, great care being taken in regard to temperature conditions, drainage, and connections. It was not considered necessary to employ the exact methods of analysis, using mercury. In some of the experiments an Orsat apparatus was used, directly connected to the exit tube by means of a two-way cock, as shown in Fig. 2.
Approximately 30 gm. of briquet material were used in each experiment, yielding about 3000 c.c. of gas. When the gas flow ceased, the furnace was cooled, nitrogen being passed meanwhile. In later work it was found that when nitrogen, or other gas, was passed through pyrogallic solution, or through both pyrogallic and phosphorus, traces of oxygen-remained, which acted on zinc at a temperature between 500° and 700° C. In the above experiments, any traces of oxygen would perhaps be taken up by the carbon of the briquets, but the very slight coatings found on some zinc globules may have been due to this small amount of oxygon; in experiments, described later, conducted in apparatus made entirely of graphite, but not tight against air, some of the zinc was decidedly coated. It is evident from the equilibrium conditions of the reaction
Zn + CO2⇔ZnO + CO,
in the presence of carbon, that, when the temperature will permit the formation of dioxide from carbon and oxygen, zinc oxide may form simultaneously.
Table 1 gives the composition of the gases from the distillation of the zinc-ore briquets, under the conditions above described. In all of the experiments using nitrogen as the displacing gas (No. 7 to 16 incl.) the zinc was essentially mirror bright, a slightly coated globule sometimes being found. In the experiments in which hydrogen was the displacing gas (No. 18 and 19), the zinc was coated with the characteristic gray coat, and in some cases zinc oxide crystals were found.
Nitrogen
In the experiments with nitrogen as the displacing gas (No. 7 to 16,inch), it will be noted that the distillation products contain appreciable percentages of nitrogen. The quantity is too great to be attributed to nitrogen left in the tube, since practically all of this should be displaced by the gas stream from the distillation. To make certain of this point, hydrogen (made from zinc and dilute sulphuric acid and purified as indicated in Table 1) was used as the displacing gas. The distilled gases still contained nitrogen in quantity; hence it must be concluded that nitrogen is a normal gas product, of zinc distillation. This is also true of hydrogen, although R. G. Max Liebig, ascribes both of these gases to diffusion into the retort from the combustion chamber. That diffusion into the retort occurs is unquestionable, as will be shown later; but in the present investigation it seems certain that this possibility was excluded.
In the distillation of coal and the manufacture of coke in byproduct ovens, nitrogen is recognized as a normal constituent of the resultant gas. O. Simmersbach has shown that the gas coming from Kopper’s ovens at the end of the coking period, at a temperature of about 1100° C., contains 15 to 17 per cent, nitrogen, and 60 to 70 per cent, hydrogen, the nitrogen being higher at the end of the coking period than at any other

Notes.—The displacing gas was commercial nitrogen in tests No. 7 to 10, inch; and hydrogen in tests No. 18 and 19. For analysis of the gas, the burette and Hempel pipettes were usee in tests No. 7 to 12, inch; and the direct-conuected Orsat apparatus in tests No. 10 to 19, inch
time. Coke always contains nitrogen, as high as 1.84 per cent, being found in English coke. This nitrogen is not expelled completely except at very high temperatures. In experiment 12c, at the very end of the distillation, at a temperature of about 1500° C., the nitrogen was found to be high. This sample was carefully taken when the gas flow had become very slow, and represents the product at the very end of distillation, or even after distillation was complete. The high nitrogen is not to be explained by the supposition that more nitrogen is given off at this time, but rather by the fact that practically no other gases are being evolved, nitrogen being the last gas to be given off. In discussing the presence of nitrogen in the distillation gases, it must be borne in mind that hard coal-tar pitch was used as the binder for the briquets. Since, the briquets were baked at a temperature of about 500° C. before distillation, this pitch was converted into a coke, but still contained some heavy hydrocarbons, which break down on further heating. Pitch contains somewhat less nitrogen than coke. It is evident that the remarks on nitrogen also apply to the distillation process when coal is employed as the reducing agent.
Hydrogen and Hydrocarbons
The reduction of zinc oxide by hydrogen according to the reaction
ZnO + H2 ⇔ Zn + H2O
undoubtedly takes place at a markedly lower temperature than the reduction by carbon, and in this experiment reduction by the hydrogen began before the gas flow due to the carbon reaction became pronounced. In the hydrogen reduction, equilibrium conditions demand increasing concentration of hydrogen with increasing temperature; hence as the hydrogen supply decreased, since it was cut off when the furnace was heated, the reaction reversed itself, with the formation of zinc oxide. At the end of the experiment, water was found in the cooler portions of the tube. The action of hydrogen on zinc oxide was investigated by Percy and Deville, both of whom found that to reduce zinc oxide by hydrogen it is necessary to have a rapidly moving stream of hydrogen; that is, the water produced by the reaction must be removed and not allowed to attain any appreciable concentration, if reoxidation is to be avoided.
The presence of methane and hydrogen in distillation gases is well known. When coal is used as the reducing agent, they represent the decomposition products of hydrocarbons. This is also true when coke and pitch are used, although the amount of hydrocarbons in the reduction material is much smaller. The two gases are most abundant at the beginning of the distillation, and disappear toward the end. In the experiments described, the briquet material occupied a considerable length of the heated tube, and as all of it was not at the same temperature simultaneously; methane and hydrogen were evolved from the cooler ends of the tube even toward the close of the distillation period. This feature, may be likened to the condition in the common retort, in which the charge is gradually heated from the outside.
In view of the action of hydrogen in the reduction of zinc oxide, the question arises as to the effect, on the zinc, of the hydrogen normally present in distillation gases. This hydrogen is evolved from the charge in the presence of a large excess of carbon, and if any water does form from the reduction of zinc oxide by hydrogen, it is immediately reduced again to hydrogen, by the carbon, with the formation of CO, CO2, or both, depending on the temperature. According to a summary by Roscoe and Schorlemmer, at temperatures of about 1000° C., the reaction between carbon and water vapor produces carbon monoxide and hydrogen, with very little carbon dioxide. As the temperature is lowered, the production of carbon dioxide increases, until at 600° C. only carbon dioxide and hydrogen are produced.
The reduction of zinc from ores, by carbon, begins at temperatures ranging from 900° to 980° C., depending on the kind of ore. These figures were obtained by the author some time ago and represent the temperatures at which zinc first becomes visible in the ignited distillation gases from small vertical graphite retorts, heated uniformly to the gas exit in an electric tube furnace. The exact figures were: Mascot, Tenn., ore, 910°; Canon City, Colo., concentrate, 940°; roasted Joplin blende ore, 940°; Burma, zinc-lead ore, 980°. The reduction of zinc by hydrogen, as previously stated, occurs at a notably lower temperature, although no definite figures are available.
Carbon Monoxide and Dioxide
At the reduction temperature of zinc oxide by carbon, carbon monoxide is the normal reaction product, since, in the presence of an excess of carbon, at this temperature, only very small quantities of carbon dioxide can exist. If, however, zinc oxide is reduced by hydrogen or by methane, evolved from the charge at temperatures below 800° C., the reaction products will consist.of water, carbon monoxide, and carbon dioxide in appreciable quantity. It is well known that carbon dioxide in certain concentration is highly undesirable in zinc metallurgy, and some definite figures on this subject are given later in this paper. In the author’s opinion, the high proportion of carbon dioxide in the distillation gases at the beginning is due to the above described reactions, although some of it comes from the reduction of iron oxides in the ore, Any zinc reduced by hydrogen or methane at temperatures below the carbon reaction will be in contact with distillation gas relatively high in carbon dioxide, and will suffer oxidation, with the consequent production of blue powder. From this standpoint, reduction coal which liberates appreciable quantities of hydrocarbons at relatively low temperatures is undesirable. Hydrocarbons and hydrogen evolved after the carbon reduction temperature has been reached are not harmful.
Carbon dioxide in excess of 1 per cent, is not found in the gases distilled above the carbon reduction temperature; below that temperature as high as 13.8 per cent, was found (experiment 11c, and others not recorded here). At the end of the distillation, the proportion increases, particularly when the temperature is high (above 1400°), the monoxide suffering decomposition into carbon dioxide and carbon. This reaction may have an effect on the zinc when smelting in an arc electric furnace, in which very high local temperatures prevail. m Carbon dioxide under 1 per cent, has no effect on zinc vapor either above or within the condensation temperature range, as is shown in Part III of this paper.
Oxygen
Oxygen, except in very small quantities, is not a normal constituent of the distillation gases. A series of experiments was made to obtain information as to the accuracy of the gas apparatus used for estimating small quantities of oxygen in such gases as commercial nitrogen. Oxygen to the proportion of 0.5 per cent, and over was readily determined, but when testing nitrogen, after purification with pyrogallic solution or phosphorus, it was difficult to obtain results which could not be accounted for by slight changes in temperature, absorption by the collecting water, or absorption of other gases by the solutions employed, although heated copper showed that small amounts of oxygen were still present. It is possible that oxygen in very small quantities was present in the distillation gases, but this was improbable since zinc vapor has a very great affinity for oxygen. The oxygen recorded in samples 8a, 8b, and 12a was probably due to defective sampling.
Condition of Zinc
The zinc condensation was sharply localized in the tube almost immediately beyond the distilling briquets. In experiments 7 and 8, about, one-half of the tube was heated uniformly by gas burners to 620° to act as a condenser, but only very small amounts of zinc were found in it, practically all of it being condensed as a mass of globules in the roof of the tube and as a little lake in the bottom beneath these, all within the space of about 1.5 in. (38 mm.). This form of condensation is to be expected from the vapor-tension curve of zinc, which shows that, in a mixture of equal parts of zinc vapor and carbon monoxide, 87 per cent, of the zinc will be condensed within the temperature range of from 865° to 750° C.
In these experiments the heating conditions, were such that, immediately beyond the distillation space, the temperature rapidly dropped to between 700° and 800°, so that the zinc, for the most part, condensed here. The tubes were 7/16 in. (11 mm.) and ¾ in. (19 mm.) diameter; and the gas flow being relatively slow, about 30 c.c. per minute, the diffusion of heat into the walls permitted a sharply localized condensation of the zinc. The zinc globules were removed by a wire rod, but the little lakes of zinc adhered so firmly to the tubes that it was necessary to break them to examine the metal. In experiments 7 to 10 inclusive, the zinc was practically all mirror bright; occasionally a globule was found with a slight brown or grayish coat, not discernible to the eye; but plain under the microscope. Adhering to this coated globule were sometimes many small mirror-bright globules. At a certain period of the distillation the gas composition may have been such as to attack certain globules, or the coating may have been due to some substance, as cadmium sulphide or carbon. The coating was not the typical blue-gray coat formed when CO2 or O2 is present. Some of the globules had assumed definite crystal structure in solidifying. In that portion of the tube on the side where the displacing gas entered was sometimes found a faint yellow-white film which, when dissolved in dilute HCl, gave the odor of hydrogen sulphide, and, after oxidation with nitric acid, gave a distinct reaction for sulphate. This coating consists probably of a mixture of zinc, oxide and cadmium sulphide; the zinc oxide was probably formed by contact of zinc vapor with a small proportion of oxygen remaining in the displacing gas, while the cadmium sulphide probably vaporized as such from the charge. According to Olsen, cadmium sulphide sublimes at 980° C. In experiment 11, a small amount of zinc frost was found; it consisted of bright feathery interlaced crystals of zinc, and evidently had passed directly to the solid state from the vapor. It was not coated. In some of the experiments, on opening the tube the odor of hydrocyanic acid was distinctly noticeable.
Conclusions from Preceding Experiments
When a charge of zinc ore and reducing agent, consisting of coke and the coked residue of hard coal-tar pitch, is submitted to the distillation process in a tight system free from oxygen, the zinc is distilled from the charge and then condensed into bright metal with the presence, only occasionally, of a foreign material which might interfere with coalescence; this is probably a sulphide, and carbon, but it occurs in such small amounts as to be technically negligible. Under proper temperature conditions the condensation is sharply localized, and no so-called blue powder is formed.
The gases consist of carbon monoxide, carbon dioxide, methane, hydrogen, and nitrogen. The carbon dioxide is highest at the beginning, but rapidly decreases to below 1 per cent.; toward the end of the distillation, methane and hydrogen disappear, and carbon monoxide increases until it composes by far the greater part of the gas. Nitrogen is persistently present in considerable quantity. Carbon monoxide suffers no appreciable decomposition into carbon dioxide and carbon in passing through the condenser when heated to between 500° and 700° C. This point is again referred to in Part III of this paper.
Preliminary Investigations
The experiments recorded above, and the development of the apparatus in which they were carried out, were preceded by considerable preliminary work which revealed the fact that experimental investigation of zinc at high temperature presents difficulties, in that zinc has a very great affinity for oxygen and other oxidizing gases, even in minute quantities, and that if reliable results are to be obtained these must be excluded. An air-tight apparatus is therefore essential. Before this was achieved, some interesting results were obtained with apparatus that was supposed to be tight, but was found not to be so at the temperatures used.
Reduction in Carbon Tube
Since excess of carbon is normal in the usual distillation process, it was natural to try a hard carbon tube as the retort in an electric furnace. A hard, dense carbon tube, 7/8 in. (22 mm.) diameter with 1/8 in. (3-mm.) walls, tight against pressure by blowing, and showing no escape of gas bubbles under water, was used. Other conditions of the experiment, the displacing gas, its purification, and the sampling, were the same as those described in Table 1. A hard glass tube, ½ in. (12.7 mm.) diameter and 36 in. (91.4 cm.) long, heated to 700° to 500° C. along its length, was used as condenser. The zinc obtained from these experiments was found partly in the carbon and partly in the condenser tube, being rather widely disseminated. It was heavily gray-coated, even in the carbon tube, although it had run together quite well. Zinc dust and some oxide were found in the condenser, and zinc dust was carried into the water bottle. The gas had the following composition, in percentage by volume:

‘The system was practically tight, and during the passage of the displacing gas gave no evidence of leaks. At the end of the experiment, the tube was found to be slightly porous when subjected to considerable pressure. The results are therefore explained by the diffusion of air into the tube, as is evident from the high nitrogen contents. During the experiment the interior of the tube was under a pressure of about 4 in. of water. It is interesting to note that zinc was oxidized simultaneously with carbon, by the infiltrated oxygen.
Reduction in Graphite Retort
In order to work with larger quantities of briquet material, a graphite retort was constructed of Acheson graphite, to the design and dimensions shown in Fig. 3. The apparatus included the retort R; the condenser
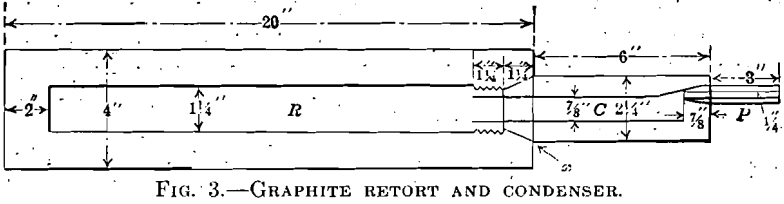
C; and the prolong P. The condenser was fitted to the retort by a screw-cone joint made tight with stove cement, and the prolong was attached to the condenser by a tight cone joint. The condenser and retort were heavily coated with Johns-Manville high-temperature cement. A glass tube was cemented into the prolong, and the distillation-gases were passed through water. The apparatus was heated to the distillation temperature in a large muffle. The gas flow was fast and free during most of the distillation, escaping against a head of 5 in. (12.7 cm.) of water. The sample was taken by inserting the discharge tube directly into the rubber connection of the gas burette.
The charge consisted of 334 gm. of briquets, which, after distillation, weighed 221 gm., a loss of 33.8 per cent, indicating complete distillation, from long experience with this type of material. The amount of zinc recovered was 23 gm. the loss being 38.8 gm. this was not lost with the gases, for practically none was evident here, but must have been due to absorption and diffusion. The gas composition was as follows, in percentage by volume:
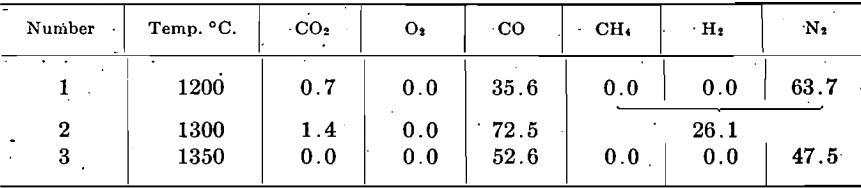
The temperatures were taken by a Pt-Rh thermocouple on the outside wall of the retort at the middle, the retort being uniformly heated throughout its length. The absence of hydrogen and methane is explained by the fact that the gas samples represent the latter part of the distillation and these gases had been expelled.
Experiment with Blue-powder Briquets
Another experiment, in the same apparatus, using briquets made from coke, coal-tar pitch, and high-grade blue powder, gave interesting results. Briquets of this composition should yield very little gas, since there is practically no reduction, and zinc vapor is the only product of the distillation. There was a constant flow of gas from the retort during the distillation, and at a temperature of about 1000° C. its composition was as follows, in percentage by volume:
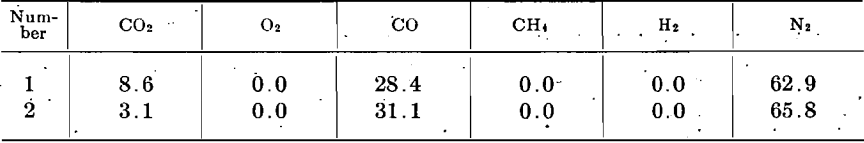
This is essentially the composition of producer gas, and is explained by the infiltration of air into the retort, the oxygen uniting with the carbon of the retort. The fact that the gas flows freely from the retort is explained by the increase in volume due to the reaction, as follows:
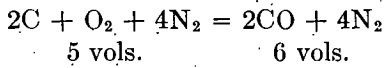
The zinc obtained in these experiments was partly bright, but mostly gray coated. Considerable blue powder was found in the condenser, although this was heated to the proper temperature by conduction from the retort. The oxidation of the zinc can be explained only by the action of oxygen, since in most cases the carbon dioxide was not high enough to react with zinc, as will be shown later.
Conclusions from Preliminary Experiments
The results obtained with the carbon tube and the graphite apparatus point to some interesting facts in distillation. At first sight, it would appear that such an apparatus must be tight, particularly the retort, which is made of dense graphite, with walls 1.375 in. thick, but experience proved that diffusion took place freely. Further, in spite of the fact that the retort material was carbon, the zinc was partially oxidized and blue powder was formed, although the temperature conditions for proper condensation to liquid zinc were maintained. This shows that when oxygen comes in contact with zinc vapor, even in the presence of carbon, at a temperature above 1000°, oxidation occurs. From the standpoint of good condensation of zinc, a tight distillation system is essential; not only must there be no openings into the system, but the material from which it is constructed must be proof against the powerful diffusion forces at work. It seems probable that much of the difficulty experienced in recovering a high percentage of liquid spelter, in the common retort process, is due to porous retorts and condensers. That there is considerable gas pressure outward from the retorts and condensers means but little when diffusion forces are at work.
These remarks apply with still greater force to the electric zinc furnace of the arc type, and it would be remarkable if such furnaces as have been used did not yield large percentages of blue powder. This subject is again referred to later in this paper.
Equilibrium of Reaction ZN + CO2 ⇔ ZNO + CO in Temperature Range of Condensation
That zinc is oxidized by carbon dioxide is well known, but the author was unable to find definite data in the literature. The work described in the first part of this paper proves that the “normal” gases arising from distillation, and present during the condensation, containing up to approximately 1 per cent, or slightly more of CO2, have no deleterious effect on condensation. Under certain conditions, more CO2 may be present, as in the case of early reduction by hydrogen or hydrocarbons, or by the decomposition of carbon monoxide.
Zinc oxide is reduced to zinc by carbon monoxide at 600° C. and zinc is oxidized by CO2 at red heat, with the formation of CO, this gas constituting over 90 per cent, of the gas issuing from the experimental tube. One of the methods of making CO gas, given in laboratory manuals, is to pass CO2 over heated zinc.
A. Lencauchez states that at white heat ZnO is reduced to Zn by CO, but that as the vapor and gases of the reaction pass to the cooler portions of the tube, the CO2 reoxidizes the zinc. He also states that blast-furnace gas containing 24 per cent. CO and 12 per cent. CO2 readily reduces ZnO at white heat, but as the temperature falls to cherry red (which he states is equivalent to 1200° C.) the CO2 reoxidizes the zinc vapor. These statements indicate that in the reaction
ZnO + CO ⇔ Zn + CO2
equilibrium requires an increasing concentration of CO2 with increasing temperature, and a decreasing concentration of CO, the reducing agent, with increasing temperature. This is the opposite condition to that prevailing in the ZnO-hydrogen reaction, and most other reactions involving the reduction of metallic oxides by CO. In the experiments described below there was nothing to indicate that the nature of the ZnO- CO reaction differed in this respect from the ZnO-hydrogen reaction.
Testing Method
A piece of pure bright zinc, in a porcelain boat, was placed in a ½-in. (12.7-mm.) hard glass tube 36 in. (91.4 cm.) long, heated in a gas combustion furnace having a mica sheet immediately over that part of the tube containing the zinc, so that the condition of the metal could be readily observed during the experiment. Purified mixtures of CO and CO2 were passed, and the effect on the zinc was noted. The temperature was measured by a Pt-PtRh thermocouple, having its junction placed directly over the center of the zinc and touching the outside of the glass tube. The temperature could be held steadily at any point in a range from 500° to 700° C. The vapor tension of zinc is such that, within the temperature range adopted, zinc vapor in considerable quantity formed in the tube and was in contact with the stream of gas. This zinc vapor condensed in the cooler portions of the tube, and in part on the cooler sides of the porcelain boat. The deposited metal could afterward be examined under the microscope, which made it easy, to distinguish sharply between bright metal, oxide, and coated metal. When oxide formed, it would partially deposit on the upper side of the tube immediately above the head of the porcelain boat, and also further along; but when the composition of the gas was such that no oxide formed, this head deposit would be absent, and bright zinc metal would condense in globules, and as a mirror, above the end of the boat, and further back in the cooler portion of the tube.
Fig. 4 shows the arrangement of the apparatus as used in the final successful experiments, after considerable work had been done to ascertain the proper conditions for success. At first, carbon monoxide, made from potassium ferrocyanide and sulphuric acid, was passed directly into the gas storage S, which also received the carbon dioxide made from pure marble and dilute HCl. The gas storage bottle was graduated and any desired mixture could be made. The mixed gases were purified by passage through water, phosphorus, cone, sulphuric acid, and anhydrous calcium chloride. Under these conditions, carbon monoxide free from carbon dioxide formed coatings on the zinc, and the difficulty was ascribed to small quantities of SO2 in the gas, derived from the ferro-cyanide and sulphuric acid reaction. The presence of sulphur was proved in the coatings, which consisted of zinc sulphide and oxide. The purification system was then modified by the addition of concentrated potassium hydrate solution between the CO generator and the gas storage, and the insertion of N/20 iodine solution between the water wash and the phosphorus bottle; also when pure CO was being used, by the further addition of KOH solution between the phosphorus and the conc. H2SO4 bottle.
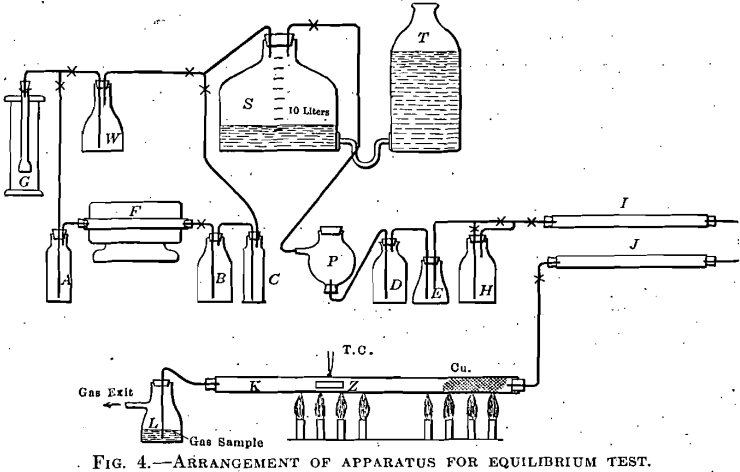

The difficulty still persisted, and coatings containing sulphur were formed. Further investigation showed that very minute quantities of sulphuric anhydride mist, originating in the CO generator, passed unaltered through the long purification train and attacked the zinc. The ferrocyanide and sulphuric acid method of making carbon monoxide was therefore abandoned, and the method of passing CO2 over charcoal heated in an electric tube furnace was adopted. The CO2, made from marble and dilute hydrochloric acid, was passed through Na2CO3 solution, over the heated charcoal, through pyrogallic solution, and concentrated KOH solution, to the storage bottle. From the storage it passed through phosphorus, iodine solution, water, cone. KOH, (in case pure CO was being used), then, through anhydrous calcium chloride, and phosphorus pentoxide. The white zinc oxide deposit still formed, but no sulphur was now present. The formation of oxide was due to minute quantities of oxygen which, were not removed by the pyrogallic solution and phosphorus, although the phosphorus was active, no inhibiting catalyzers being present, and the gas stream did not exceed 30 to 40 c.c. per minute.
The final purification train then adopted was as last described, with the addition of copper gauze and shredded copper placed in the glass tube, near the end where the gas stream entered, and the replacement of the iodine solution by Na2CO3 solution. This train removed the troublesome foreign constituents from the gas stream, and further experiments gave consistent results.
The difficulties encountered are briefly outlined above for the purpose of showing how readily errors can be made in the investigation of a metal like zinc, which has such strong chemical affinity at the temperature of condensation.
For determining the composition of the gas mixture, the following method was used. The pure gas, or the mixture, would be accumulated in the storage bottle, which was then thoroughly shaken to insure a uniform gas. The copper was then heated to redness, and the gas was passed through the train at the rate of 30 to 40 c.c. per minute, until from 2500 to 3000 c.c. had been used. The sample was then taken in the manner described in Part I, an accurate glass stopcock burette, graduated to 0.1 c.c., being used in connection with Hempel gas pipettes for analysis. The analysis thus obtained represented the composition of the gas in contact with the zinc. The gas burners under the zinc were then lighted and the experiment carried through to the end. Aside from CO and CO2, the gas contained usually between 5 and 8 per cent, of nitrogen and fractions of 1 per cent, of H2. The source of the nitrogen is the charcoal, small amounts probably being due to the marble. The hydrogen comes from decomposed water vapor. The presence of these gases is not detrimental, since they represent normal constituents of distillation gases, which do not affect condensation.
Conclusions from Equilibrium Investigation
Table 2 contains the experimental data and Fig. 5 is the equilibrium curve drawn from these data. The gas composition is recalculated on the basis of a mixture consisting of CO and CO2 only, omitting the nitrogen and hydrogen content. The results may be summed up in the statement that, within the condensation range between 500° and 700° C., distillation gases must not contain more than 2.5 per cent. CO2 if oxidation of the zinc is to be avoided entirely; when present between 2.5 and 5 per cent.
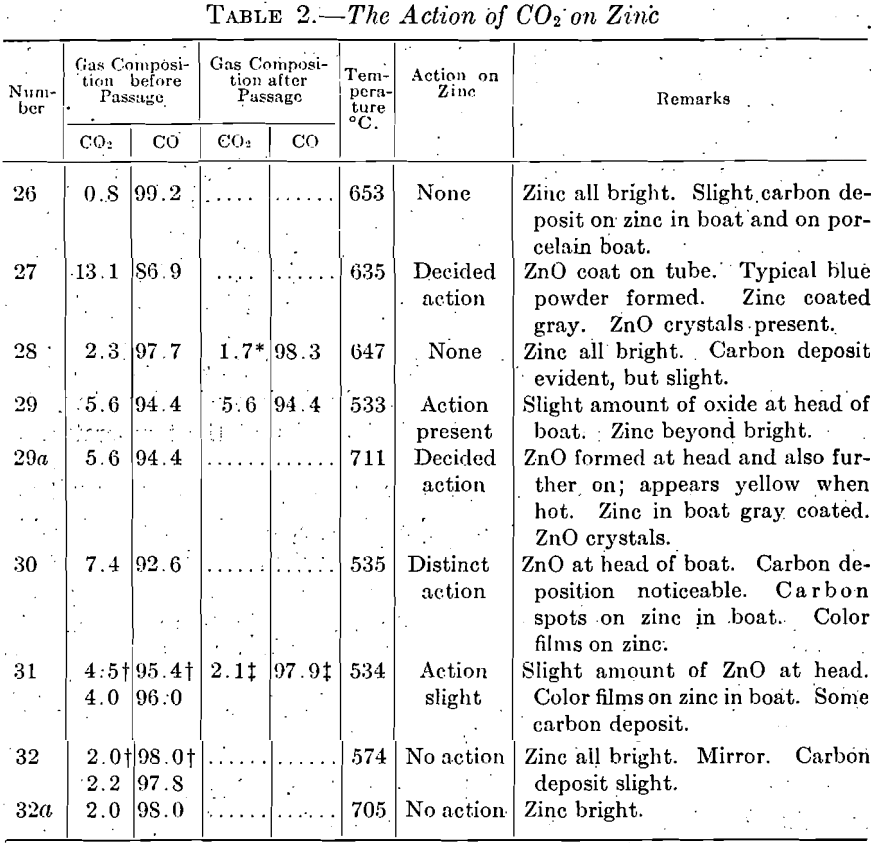
the action of CO2 is comparatively slight; when more than 5 per cent, is present, oxidation is pronounced, and blue powder begins to form.
That decomposition of CO occurs in the condenser tube is evident from the slight carbon deposit formed, but it is small and its detection by analysis of the gas going, in and coming out is uncertain, as the slight differences in composition are not readily determined. This confirms the data obtained from the experiments on composition of distillation, gases, previously described, based on experiments 7 and 8. It is therefore concluded that in a condenser system in which there is a movement of gas not under normal speed (i.e., one at which condensation of zinc vapor can readily take place) the decomposition of CO is not sufficient to interfere with condensation.
The reaction 2CO⇔CO2 + C has been thoroughly investigated, and it need only be stated here that it proceeds from left to right much more rapidly at 500° than at 700°, and that the catalytic agents iron or nickel have a great influence on the speed of the reaction. It is advisable, therefore, in the design of a condenser, not to use structural material containing iron, or iron itself, in its interior; neither should the gas velocity be too slow, nor should the condenser be held at too low a temperature (450° to 500° C.). The last difficulty may be overcome by the use of a primary and a secondary condenser, the first being larger and held at the higher temperature range, while the second is smaller and held at the lower temperature range.

Conditions of the Zinc in Equilibrium Experiments
In the preceding experiment, a relatively small amount of zinc vapor was contained in a large volume of gases. When the CO2 was below the quantity necessary for attack on the zinc, the metal condensed beyond the heated zone of the tube in minute drops and globules firmly adherent to the glass, and all were mirror bright. Further along, a metal mirror would appear. In most of the condensing region the temperature was below, the melting point of zinc. The general appearance was very similar to that of water vapor condensing on a pane of glass. There was no loosely adherent powder, but toward the very end of the tube there were minute shining crystals of zinc. The zinc in the boat was partly bright and in part covered with a brown film and dark brown spots which were taken to be carbon.
When CO2 was present in sufficient amount to attack the zinc readily, loosely adherent gray coatings and deposits formed above the boat, and further along, typical blue powder, accompanied by bright metal globules and white oxide films.
In the absence of interfering substances, the condensation of zinc vapor into liquid metal drops, and the coalescence of these drops into a bath of metal, present no particular difficulties. The coalescence of the zinc drops is governed by surface tension and related forces, and if these could be diminished, condensing surfaces might be made more efficient. The subject presents an interesting field for investigation.
Requisites for Successful Condensation
The object of the experimental work detailed in this paper was to determine certain fundamental facts in the distillation and condensation of zinc, in order to find an answer to the question—Are any products formed during the distillation of zinc ore and the subsequent condensation of the zinc vapor which interfere with the condensation to liquid metal; if so, what are they, and in what proportion are they harmful?
The results of the investigation show that under proper conditions there is nothing to interfere with the condensation of the zinc to liquid metal, but that the latitude within which operations may be carried on is not wide. One requisite is that the distillation and condensation systems must be tight and impervious to air. This, of course, has been well known, but the fundamental reason for it has not been recognized. Many of the electric zinc furnaces that have been experimented with, or proposed, suffer from this vital defect, the arc furnace being particularly defective on this point. If operated continuously, by feeding ore and reduction carbon, and discharging slag, there will be regions in the furnace, in the cooler portions above the smelting arcs, where carbon dioxide will form in considerable proportion, according to the principles discussed in this paper. For an electric zinc smelting furnace to be successful, it must be able to obtain a uniform temperature rapidly throughout its whole distillation chamber; certainly no part of the chamber or its contents should be below 950° to 1000° C. From the standpoint of the condensation of vapor, it is not possible to distil at 1200° C. in one part of the chamber, while the products of distillation come in contact with ore charge at below 1000° C. in another part, and at the same time condense a large proportion of the vapor to liquid spelter.
