Table of Contents
Most papers on xanthate have dealt with principles rather than practice. On the assumption that many millmen are interested in knowing where and in what manner the xanthates are being used in mills other than their own, this paper will summarize the results of a recent survey of North American plants. A brief background story and a description of the xanthates will be followed with the results of that survey; then a few observations will be made on storage, feeding, and ore testing.
The first xanthate was made in 1822 by Zeise of the University of Copenhagen. With commendable scientific detachment (or perhaps a bad cold) Zeise ignored a more pervading quality of the substance and named it for its color, after the Greek word “xanthos,” meaning yellow. During the first 80 years of its recorded existence xanthate found use only in small quantity as an insecticide; after the turn of the century there was some further demand for it in the vulcanization of rubber. It was not until 1922, by coincidence the centennial of Zeise’s discovery, that C. H. Keller of San Francisco, seeking sulphidizing agents, recalled xanthate in connection with his experience years earlier when he had seen this material used in Europe to combat grape phylloxera. His successful tests with the new flotation reagent culminated in the basic xanthate patent (U. S. 1,554,216) in 1925.
There is general agreement, nonetheless, that the introduction of xanthate was momentous and opportune, for as an adjunct to the other important developments of that “golden age of flotation” it frequently supplanted frustration with success. Those who struggled with selective flotation during its infancy will recall the skepticism and then the profound relief with which the first of the xanthates was welcomed. Welcomed they were indeed, for in a very short time the xanthates attained prominence as collectors for the sulphide minerals, for some of the
oxidized minerals of copper and lead, for such elemental metals as gold, silver, copper, and such nonmetals as graphite and sulphur. While this accomplishment was of prime importance there was also a secondary role for the new reagent; to the research scientists who sought a complete theory of flotation it afforded a precise and convenient tool. Consequently, in much of the valuable work on the theory of flotation that has been published during the past 20 years xanthate collectors have been employed, principally because they are specifically suited to the control and measurement required in such investigations, but also because their wide use has made them a logical standard of comparison.
Description of the Xanthates
Xanthates are alkyl sulphur derivatives of carbonic acid, known as dithiocarbonates. Strictly, a xanthate is any salt of xanthic acid, but the xanthate flotation reagents are specifically the reaction products of carbon disulphide, an aliphatic alcohol, and either caustic soda or potash. The general formula is ROCSSM, in which R stands for an alkyl hydrocarbon radical and M for an alkali metal. Many xanthates have been compounded on the basis of this reaction, some of them only laboratory curiosities, others possessing desirable qualities in varying degree, but eventually the number of alcohols (including their homologues) was narrowed to four: the ethyl, isopropyl, butyl, and amyl.
The first xanthate on the market, the potassium ethyl xanthate initially used at Anaconda, was a relatively pure material but its manufacturing process resulted in a mother-liquor by-product. Improvements in the methods of manufacture soon resulted in the elimination of this by-product and a dry material of high purity and stability was produced. Then followed the sodium ethyl xanthate, a number of potassium amyl xanthates, several butyl and isopropyl xanthates, and finally a hexyl xanthate. The last was discontinued after only a short period of manufacture. At present only the eight xanthates listed in Table 1 are available in commercial quantities.
Applications
Generalizations are not looked upon with favor by the hard-bitten millman unless they are supported by actual performance data. For this section of the paper, therefore, statistical data covering the use of flotation collectors (and other relevant matter) in practically all of the sulphide ore-flotation mills of over 100 tons daily throughput in North America were tabulated and analyzed. These will be discussed here under the heads, Differential Lead-zinc Ores (61 mills), Copper (32), Differential Copper-zinc (8), Straight Lead and Zinc (13 and the Tri-State), Gold (22), and Miscellaneous (6). A few omissions occur, resulting from the lack of authentic information, but the data are substantially factual and complete.
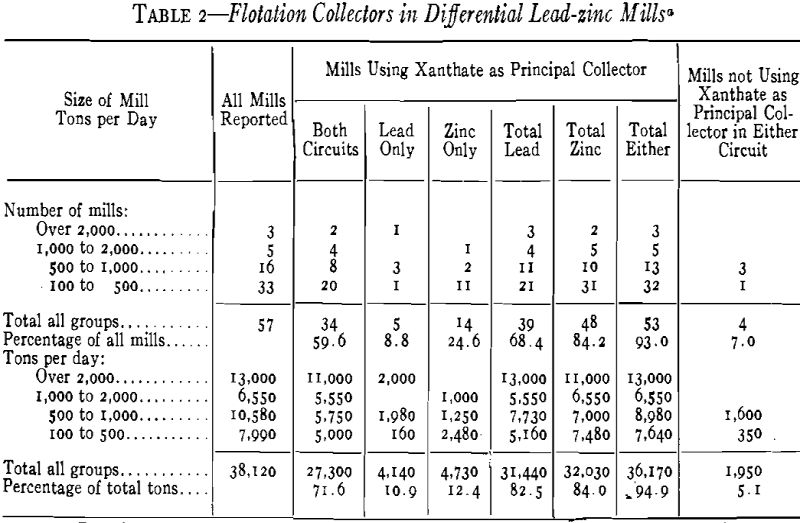
Differential Lead-Zinc Mills
Sixty-one mills are included under this heading; 57 of these use the differential process of floating a lead concentrate first and then a zinc product, while the other four float a bulk concentrate of all sulphides, following with differential separation of this concentrate. The main group will be discussed first. Of these 57 mills, S3 use xanthate as the principal collector in one or the other circuit or both and mill 95 pct of the lead-zinc ore now treated by this process in North America (Table 2). In 60 pct of the mills (72 pct of the tonnage) xanthate is the principal collector in both circuits; in 68 pct of the mills it is the principal lead collector (82 pct of the tonnage); in 84 pct of the mills it is the principal zinc collector (84 pct of the tonnage). All mills larger than 1000 tons use xanthate as the principal collector in one or both circuits.
It is perhaps advisable to clarify the term “principal collector.” In these data we have considered xanthate the primary collector only when it is used in greater quantity than any other collector in the circuit and in most cases more than the total of all others.
Of 39 mills using xanthate as principal collector in the lead circuit:
Ethyl xanthates are used in 19 mills (48.7 pct)
Mixtures ethyl and amyl or pentasol are used in 6 mills (15.4 pct)
Butyl xanthates are used in 6 mills (15.4 pct)
Amyl or pentasol xanthates are used in 5 mills (12.8 pct)
Isoprophyl xanthates are used in 3 mills ( 7.7 pct)
In the zinc circuit the preference for the higher-alcohol xanthates is less marked than in the lead, again confirming Wark and Cox, who found amyl xanthate not so effective as ethyl xanthate in separating sphalerite from pyrite except at high alkalinity. Nevertheless, mixtures of C2 and Ct xanthates have been found advantageous even at low alkalinities.
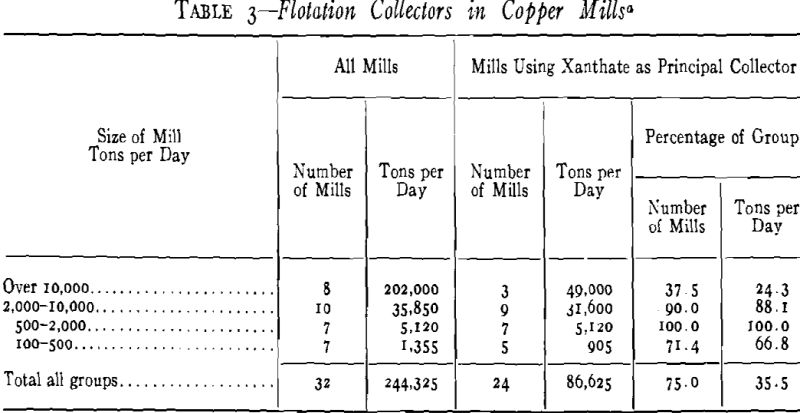
Copper Mills
The porphyry coppers may be roughly divided into two classes, the straight chalcocite ores and the ores containing chalcopyrite. The former ores may be considered more difficult as regards grade of concentrate, because pyrite persists in the froth, partly because it is often coated or stained with secondary copper, and partly because its association with copper mineral is too intimate for physical separation even after minute reduction by regrinding of the primary concentrate. As a result the finished concentrate rarely runs higher than 40 pct copper, although chalcocite itself contains nearly 80 pct copper. Xanthates function well on this type of porphyry ore because potency and speed of collection are the essential properties, and the only purpose of a slower or weaker collector is to reject a low grade, but still valuable, portion of the recoverable mineral when grade of concentrate is unusually important.
In the second class of porphyry ores, the chalcopyritic, the problem is also to separate the copper minerals from pyrite, but in this case there is usually less activation of the pyrite or intimate association of it with copper minerals. Xanthates are used on this type of porphyry ore because they function well in the higher lime alkalinities that often are necessary for maximum selectivity between the pyrite and chalcopyrite.
Differential Copper-Zinc Mills
Eight mills in North America are reported to be making the differential copper-zinc separation. Xanthate is used in either the copper or the zinc circuit at seven mills, and in both circuits at four mills. Lime is more commonly used than sodium carbonate as the pH modifier in the copper section. For deactivating sphalerite and pyrite (or for preventing activation) cyanide is used in six mills; with zinc sulphate in three of these, sodium sulphite in two, and by itself in one.
The separation of chalcopyrite from sphalerite is one of the most difficult, because copper dissolves during grinding and flotation, activating the unwanted minerals. Wark and Cox have indicated that the formation of the copper-bearing film on sphalerite is easier to prevent than to counteract, hence the depressants and pH modifier should be on the ore when it is first pulped with water. They also suggest that this counteraction is best applied before xanthate is added, since the copper-bearing film on sphalerite is more soluble than the dual copper-and-xanthate film.
