Table of Contents
It is essential for the assayer to be assured of the fact that his reagents are pure, or at least to know to what extent they are impure and what the impurity consists of. For this reason it is necessary to examine lots of reagents from time to time, as they come into the laboratory, by approved chemical methods, to determine their purity. Sometimes reagents or fluxes, as a result of being left exposed in the laboratory, become accidentally or purposely “ salted ” or contaminated with gold, silver or base-metal values. A blank assay for metals on the reagents will readily determine this. In general, it may be stated that the labeling of a chemical “ c. p.” does not necessarily make it so. Borax has been found to contain platinum.
It is necessary to determine the silver in litharge and test lead, as these two reagents frequently contain some silver, due to their being usually made from lead bullion refined by the Parkes’ or zinc-desilverization process, which leaves some silver in them. As litharge is almost invariably used in the crucible assay, and test lead in the scorification assay, any silver or, possibly, gold introduced into the results by their use must be subtracted, so as not to be ascribed to the ores. Most assay supply houses now furnish practically silver-free litharge and lead containing only traces of silver and no gold.
The method of determining silver and gold in litharge and test lead is as follows:
The following charge is weighed out in duplicate:
Litharge……………………………………………..3 assay tons
Sodium carbonate…………………………………20 grams
Silica…………………………………………………….7 grams
Argol…………………………………………………..2 grams
Borax glass………………………………………5 grams (as a cover)
The various ingredients are put from the scale pan on a sheet of glazed paper and thoroughly incorporated by mixing. It is essential to weigh the litharge and argol as accurately as possible with the pulp balances in use.
The incorporated charge is then transferred to a 20-gram crucible, a shallow cover of borax glass being put on top of the charge, and then fused in the muffle-furnace for from 25 to 35 minutes at a yellow heat (1000° C.). The fusion is considered complete when the charge is in quiet fusion, that is, when there is no more bubbling and boiling in the charge and when the only motion observable is that due to convection currents. The charge is then poured into an iron mold and allowed to solidify, which takes approximately 10 minutes. The lead button is then separated from the slag by the hammer and formed into a cube. It is weighed and its weight recorded in grams and tenths of a gram in the assay note-book, a definite assay number being assigned to this assay and its duplicate. The lead button is then cupeled, the cupel being first placed in the muffle for 10 to 12 minutes before the lead button is dropped into it. If the button weighs from 15 to 20 grams, as it should, it will take 25 or 30 minutes to finish the cupellation, that is, to drive off the lead. The end of this operation, in this particular instance, is denoted by the darkening of the small silver bead. The bead is then removed from the cupel after this has become cold, flattened on a small anvil with a blowpipe hammer, cleaned of adhering bone-ash from the cupel by a button brush, and weighed carefully on the assay balances, the weight being recorded in milligrams and hundredths of a milligram. The weight of the bead, divided by the number of assay tons (3) taken in the assay, gives the number of ounces contained in a ton (2000 lb.) of litharge, or the number of milligrams per assay ton of litharge. If the presence of gold is suspected in the litharge, the silver bead from the cupellation, after weighing, is dropped into a parting-cup filled with hot nitric acid (9 parts water to 1 part concentrated nitric acid, sp. gr. 1.42), which will dissolve the silver and leave the gold as a black residue. This residue is washed three times by decantation with cold distilled water, carefully dried and annealed at a red heat in the muffle; after cooling it is weighed as already described for silver. The weight of the gold is recorded and then subtracted from the weight of the original gold and silver bead. The difference in weight gives the amount of silver.
To determine the silver and gold in test lead, weigh out 3 assay tons, place in a 2.5-in. scorifier, add a pinch of borax glass, and scorify in the muffle at a yellow heat (1000° C.). As the lead oxidizes to litharge, this melts and forms a slag which, owing to the convexity of the meniscus of molten lead, falls to the side of the surface and forms the slag ring, leaving a disk of fresh lead exposed. The scorification is finished when the slag finally covers all the lead. The charge is then poured into an iron mold, the further method of procedure followed being identical with the one described for the litharge assay.
It is possible to obtain test and sheet lead with only traces of silver, and litharge practically free from silver. It is often desirable that the litharge should contain a uniform amount of silver, for whenever low-grade gold ores, deficient in silver, are assayed, silver will have to be added at some stage of the assay in order to insure parting, or the complete separation of the gold from the silver. In assaying very low-grade gold ores, in which practically only gold is present, the final bead might be so small as to sink into minute cracks in the cupel and thus be lost. The addition of silver in this case, either by adding it in the metallic state or by its presence in the litharge, obviates this difficulty.
Litharge will frequently contain from 0.20 to 0.32 mg. of silver per assay ton. It is, however, not safe to assume the above figures. The test lead ordinarily bought from the supply houses contains only traces of silver.
Assaying includes all those operations of analytical chemistry which have for their object the determination of the constituents of ores and metallurgic products. Three methods are used:
- Fire assaying (dry methods);
- gravimetric analysis (wet methods);
- volumetric and colorimetric analysis (wet methods).
This work treats of fire assaying only, with a few exceptions. The quantitative determination of the following metals is discussed: gold, silver, platinum, etc., lead, antimony, bismuth, tin and mercury; chiefly, however, gold and silver.
Fire assaying comprises the separation of the metal sought from the other components of the ore, by heat and suitable fluxes, and then the weighing of it in a state of greater or lesser purity.
Gold and silver are determined in their ores, or metallurgic products, by collecting them with lead, forming an alloy, which may be accomplished either by the crucible or the scorification fusion, the lead being then driven off by cupellation, and the resultant bead of the gold and silver alloy weighed. The separation of gold from silver is accomplished by parting in most instances with nitric acid, rarely by sulphuric acid.
In order to successfully collect the precious metals by means of lead, it is essential that the ore be mixed with suitable fluxes, so that in fusion the ore is thoroughly decomposed chemically, and a liquid slag of the proper constitution produced, enabling the lead with its alloyed gold and silver to settle from the slag by gravity, thus affording a ready separation.

1. Litharge is acted on in the crucible by reducing agents, such as charcoal, etc., and metallic lead produced as follows:
2PbO + C = 2Pb + CO2
The litharge not reduced is acted on by silica and borax glass, producing silicates and borates of lead, as follows:
PbO + SiO2=PbSiO3, etc.
Litharge melts at 884° C.
2, 3. Sodium carbonate is decomposed by heat in the crucible, as follows, at high temperature not usually reached in assaying:
Na2CO3 = Na2O + CO2
Or, in the presence of silica, at lower temperature,
Na2CO3 + SiO2 = Na2SiO3 + CO
The Na2O, with silica, forms sodium silicates, as Na2SiO3, etc., which are very fusible. It also possesses the property of readily forming sulphides and sulphates and, in the presence of metallic Fe, of freeing lead in the charge from sulphur.
Na2CO3 melts at 814° C.
Assayers frequently use sodium bicarbonate in place of calcined sodium carbonate, particularly in the United States, on account of its lower cost. Thus while refined sodium carbonate costs 8 cents per pound, sodium bicarbonate costs but 3 cents per pound, at commercial centers. When the salts are calculated to the basis of the base (Na2O) contained, the difference in cost is not so wide, still the bicarbonate is cheaper. Nevertheless it is preferable to use the carbonate, since the great amount of gas evolved in the decomposition of the bicarbonate is apt to cause mechanical losses in the assay. Crude sodium carbonate or soda-ash may be used costing about 2 cents per pound. In the crucible under the influence of heat the bicarbonate decomposes as follows:
2NaHCO3 = Na2CO3 + H2O + CO2
4. Potassium carbonate acts in a similar manner to sodium carbonate. It melts at 885° C.
5. Silica is a powerful acid flux and combines with the metallic oxides or bases present in the charge to form the slag, which is mainly composed of silicates. It is present in most ores in considerable quantity, ranging from small amounts in basic ores to the main bulk of the ore in quartz ores. It melts at 1775° C. (Quartz).—(Roberts-Austen, 1899.)
6. 7. Anhydrous boric acid (B2O3), Borax (Na2O.2B2O3, 10H2O), and Borax glass (Na2O.2B2O3) or anhydrous sodium bi-borate. Boric acid readily forms borates on fusion at comparatively high temperature with lithium, potassium, sodium, and silver oxides, generally forming orthoborates (3Na2O.B2O3). Boric acid does not readily dissolve silica, but sodium or potassium meta-borate (Na2O.B2O3, K2O.B2O3), formed probably during the fusion of borax glass, or sodium bi-borate (Na2O.2B2O3), with bases, will readily dissolve silica, as well as alumina and chromic oxide. The alkaline meta-borates are markedly volatile when molten and deliquesce in the air.
Borates are classified as follows:Ortho-borates, e.g. 3CaO.B2O3; pyro-borates, e.g., 2CaO.B2O3; sesqui-borates, e.g., 3CaO.2B2O3; meta-borates, e.g., CaO.B2O3; and bi-borates, e.g.,CaO.2B2O3. The following borates are of interest to the assayer: Magnesium ortho-borate, 3MgO.B2O3; magnesium pyro-borate, 2MgO.B2O3; the corresponding borates of nickel and cobalt; the ortho-,pyro-, meta- and bi-borates of calcium, strontium and barium. Lead oxide forms glasses with boric acid and borax, of which PbO.B2O3 is hard like flint glass, and 3PbO.B2O3, may be softened in boiling oil. Other substances which may not be compounds are: 3ZnO.2B2O3; 3ZnO.B2O3; MnO.B2O3; 3MnO.B2O3; 3MnO.2B2O3; CuO.B2O3; 3Cu2O.2B2O3; and 3B2O3.2FeO.2Fe2O3. Bismuth, antimony and arsenic also form borates.
Borax and borax glass are fluxes used frequently by assayers. They are considered acid fluxes, but it will be noted from the above that they have the power of dissolving silica and alumina and will hence corrode crucibles. They can be used to flux silica to a certain extent, a use, however, to which they are not put. Sodium bi-borate has the property of passing gradually from the liquid to the solid state (amorphous) and vice, versa, under ordinary conditions with no definite freezing- or melting- point. It can be made to crystallize or freeze at a definite temperature only under the influence of vibration from rapidly repeated shocks. Crystallized sodium bi-borate melts at 742° C.
The use of borax glass as a flux to form easily fusible borates with metallic bases is dependent upon the liberation of boric acid from the bi-borate, in the presence of the free bases. What particular borates form is largely a question of temperature attained. The use of much borax gives rise to hard stony slags, very tough, from which the lead button separates with difficulty. Often a film of lead will adhere to the slag, causing mechanical loss. Slags containing much borax will often fly to pieces suddenly, especially when touched with a sharp instrument, while cooling. This is due to devitrification of amorphous glassy borates and the formation of definite crystallized borates.
In fluxing ores containing zinc it is to be noted that boric oxide, either alone or mixed with one-half its weight of borax, will flux zinc oxide into a very fluid slag, which is, however, very corrosive to clay crucibles.
8. Fluorspar is occasionally used in assaying. It melts at a comparatively high temperature, 1330° C., but when fused is very thinly fluid. The greater part of it remains unchanged throughout the fusion, and hence its lime cannot be considered as available for fluxing silica. It gives the slags containing it a stony appearance. Owing to its great fluidity, it has the property, shared by soda and litharge to some extent, of holding in suspension unfused particles, thus still making a fluid slag. Where the decomposition of the ore to be assayed is essential, as it is in most cases, its use is not to be advocated.
9. Lime is used either as the carbonate or as the oxide or hydrate. In the crucible it is converted into oxide, the carbonate beginning to lose its CO2 at 800° C. In itself it is extremely infusible (1900° C.; Hempel, 1903), but with silica, when joined with other bases and in moderate quantities, it makes very desirable slags. It is found in many ores. Magnesia acts in a similar way. Its melting-point is 2250° C. (Hempel, 1903.)
10. Hematite, or natural ferric oxide, and limonite, are of frequent occurrence in ores, and are sometimes added as a flux. Ferric oxide has a high melting point, about 1560° C. In the crucible it is converted by reducing agents, such as argol, charcoal, etc., to ferrous oxide (FeO), and then unites with silica to form silicates. The fact that it is reduced to ferrous oxide, conversely gives it an oxidizing power. Manganese oxides acting in a similar way are also frequently found in ores. Alumina, Al2O3, is often found in ores, and unites with silica to form silicates. It has no oxidizing power. Al2O3 melts at 2010° C. Kanolt (1912.)
11. Test lead and sheet lead are used chiefly in the scorification assay and in cupellation. In both of these operations the lead is oxidized by the oxygen of the air (2Pb + O2 = 2PbO) to litharge. In the scorification assay part of this PbO volatilizes; the greater part becomes fluid and holds in suspension and solution other metallic oxides derived from ores, thus forming what is termed an oxide slag. In cupellation, part of the lead is volatilized as PbO, and part is absorbed by the cupel as PbO. Lead melts at 326° C.
12. Argol is a crude bitartrate of potassium, separating out in wine casks, from the wine on standing. On heating, it breaks up as follows:
2KHC4H4O6+heat = K2O+5H2O +6CO+2C
The carbon and carbon monoxide set free gives it its reducing power. The K2O left acts as a basic flux.
13, 14, 15. Charcoal, coke, coal dust, sugar and flour are reducing agents by virtue of the carbon or hydrogen, or both, that they contain.
16. Lead flux is a ready-prepared flux used mainly in the assay of lead ores for lead. It has the following composition:
Sodium bicarbonate…………………………………………………16 parts
Potassium carbonate………………………………………………..16 parts
Borax glass……………………………………………………………….8 parts
Flour……………………………………………………………………….4 parts
It is also made up in other proportions.
17. Black flux is made of 1 part KNO3 and 3 parts argol, deflagrated. It is sometimes used in the tin and lead assay.
18. Black flux substitute consists of 3 parts of flour and 10 parts of NaHCO3. It is used in the tin assay.
19. The alkaline cyanides are powerful poisons and when powdering them for use as a flux great care must be taken not to inhale the dust. The mortar in which the pulverizing is done should be covered by a cloth during the operation, which is best conducted at an open window. Two kinds of commercial cyanide may be readily purchased on the market. 1. What is known as “ potassium cyanide,” but which consists of the mixed cyanides of sodium and potassium, containing varying amounts of impurities such as alkaline carbonates, sulphates, etc. The quality is expressed by the cyanogen content, in terms of KCN. Thus “98 per cent. KCN” is in common use. Without going into detail, it is to be noted that salts of this type may contain considerable impurity, although rated as “98 per cent. KCN,” and unless known to be good should not be used in the tin assay. Pure potassium cyanide, c.p. can be obtained only at a comparatively high price. 2. Sodium cyanide. This is a commercial salt that may be obtained nearly pure. When its cyanogen contents are rated at 125 to 130 per cent. KCN, it may be used with safety as a flux for the tin assay.
A sample of commercial “98 per cent. KCN,” impurities not known, had a freezing-point of 526° C., as determined in the authors laboratory.
When heated somewhat above its melting point in the presence of air, alkaline cyanide forms cyanate and then decomposes with the liberation of cyanogen. Crucibles in which it is used should be covered. The alkaline cyanides are used mainly in the assay of base metals as bismuth, lead, tin and antimony.
It is a powerful reducing and desulphurizing agent, acting as follows:
PbO + KCN = KCNO+Pb
PbS + KCN = KCNS +Pb
20. Potassium nitrate or niter is used as an oxidizing agent. With metallic lead it acts as follows:
7Pb +6KNO3 = 7PbO +3K2O + 3N2 + 4O2 (approximately).
It is frequently used in assaying to oxidize impurities in the charge, such as sulphur, arsenic, etc. It acts as a basic flux. Potassium nitrate fuses at 339° C.
Sodium nitrate or Chile saltpeter is sometimes used in place of niter, but as it deliquesces much more than the latter it is not so convenient.
Other oxidizing agents such as potassium permanganate, potassium ferri cyanide, etc., may be used in the assay of impure ores, but are more expensive and not any better. It is desirable to dry niter at 100° C. before use and then keep it in a closely stoppered bottle, otherwise it will be weakened per unit weight on account of the absorbed moisture.
21. Salt (NaCl) is used as a cover. It is very thinly fluid and is not decomposed during the fusion. It freezes at 801° C.
A Reagent may be defined as a substance that reacts, that is, brings about some chemical change.
The student will by this time be quite familiar with many reagents employed in qualitative and quantitative chemical analysis, A new class of reagents will now be considered — a class the majority of the members of which require the aid of considerable heat before they fulfill their function.
Reagents used in Fire Assaying
The following tabulation includes the most important reagents used in ‘fire’ assaying, The majority of these reagents are solids, though a few liquids are employed. The terms employed are defined as follows:—
A Reducer is a substance capable of removing oxygen from its combinations.
An Oxidiser is a substance capable of readily giving up oxygen to other substances.
A Flux is a substance which by forming fusible compounds readily brings about the fusion of otherwise refractory substances.
The terms Sulphuriser and Desulphuriser explain themselves. Also the term Solvent, molten lead. e.g. for dissolving gold.
The terms ‘Absorbent’ and ‘Cover’ will be fully explained further on, as also will be the terms ‘Inquartation’ and ‘Speise’.


Considering in detail these reagents, the first group is that of the reducers. The following equation represents the action of charcoal on litharge when subjected to a red heat in a crucible,
2PbO + C = Pb2 + CO2
Litharge and carbon yield lead and carbon dioxide. When the student comes to the practical testing of the strength of reducers he will find that charcoal is a very powerful reducer, and that it is more advisable in many cases to employ a weaker reducer, such as flour or argol. By employing a weaker reducer it is found that, owing to its greater quantity, it can be more readily and thoroughly mixed with the ore, and also that less care need be exercised in weighing the reducer, as a difference of a few grains more or less of argol makes much less difference in the weight of the resulting lead button than a similar difference in the use of charcoal.
The reducing power of flour depends chiefly on its carbon contents; and as these vary slighly with the different ‘ brands ’ of flour, the reducing power will be found to vary correspondingly.
Argol is a crude bitartrate of potassium (formula KHC4H4O6). This reducer is of much service to the assayer, being fairly constant as regards reducing power; and further, is of a convenient strength for much of the routine work of the fire-assayer.
Potassium Cyanide—(KCN).—This salt is largely used in the fire-assays of tin and lead ores, and acts as a reducer towards the oxides of these metals, cyanates being formed as shown in the equation
SnO2 + 2KCN = Sn + 2KCNO
metallic tin and potassium cyanate being formed.
The following tabulation gives the reducing powers of some of the chief reducers as well as of certain substances, such as sulphides, which are found in certain ores which come under the assayer’s notice. These sulphides act as reducers to substances such as litharge, the sulphur uniting with the oxygen of the litharge, metallic lead being deposited, and the metal of the sulphide either remains free or combines with other substances present to form a slag. When, for example, it is stated that the reducing power of a substance is 13, it is understood that one part by weight of the reducer is capable of reducing 13 parts by weight of lead from litharge. Twenty grains of this reducer mixed with excess of litharge (in a crucible and heated to a bright red) will reduce a lead button of 20 x 13 = 260 grains.
REDUCING POWERS
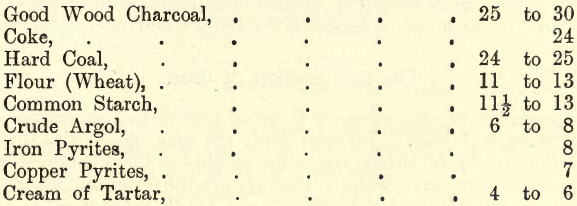
The student must remember that these figures are only approximate, and that on trial he may obtain results varying one or two units from the figures given, the reason being that such substances are rarely, if ever, obtained in a state of purity, and the impurities contained are by no means constant in different samples of the same material.
OXIDISERS
Under this heading come a number of substances capable of giving up all or part of their oxygen contents to other bodies, on the application of heat. The oxidising power of these substances may be determined by chemical calculations based on their behaviour on the application of heat. Thus we may compare the oxidising powers of Nitre (KNO3) and Red Load (Pb3O4) if we know the chemical reactions taking place between the oxidiser and the substance oxidised. If a mixture of KNO3 and metallic Pb be heated the following reaction takes place—
KNO3 + Pb = KNO2 + PbO
Heated with carbon the following reaction occurs –
4KNO3 + 5C = 2K2CO3 + 3CO2 + 2N2
Red Lead (Pb3O4) when heated above 450° C. yields up part of its oxygen thus—
Pb3O4 = 3PbO + O
The oxygen thus set free is ready to unite with and oxidise other elements present. This reagent may be used in place of litharge when dealing with pyritic ores, sulphur being oxidised, and metallic lead left as a collecting agent for gold and silver.
It must be remembered that oxidation and reduction are two processes which must often be regarded as the complements the one of the other, that is, when one substance is oxidised another substance is frequently reduced. As an illustration, the action of charcoal on red lead may be cited. The charcoal reduces metallic lead from the red oxide, and at the same time the oxygen of the red oxide oxidises the charcoal, forming oxides of carbon. Concerning these and like reactions the student will find full information in any of the well kuown text-books on the chemistry of the metals and non-metals. The student who has a fair grasp of the principles of theoretical chemistry will find little difficulty in understanding the various reactions of oxidation and reduction employed in the various processes of fire-assay.
Fluxes.—These have already been defined as substances employed to promote the fusion of refractory substances. Complex substances are formed whose melting points are much lower than those of their refractory constituents.
That the student may intelligently employ such substances, he must (1) know the approximate composition of the ores he is dealing with; and (2) this being known, he must understand the nature and use of his fluxes.
The Composition of Ores.
Some knowledge of mineralogy will be of service to the assayer in determining the nature of ores. In most cases the ores dealt with by the fire- assayer consist chiefly of either oxides or sulphides, though minerals containing tellurium or antimony, arsenic, etc., are not infrequently met with in ores.
The following brief tabulation, though not at all exhaustive, embraces the majority of ores dealt with by the fire-assayer.
I. Oxidised Ores, Acid. Basic.
II. Sulphide Ores.
III. Ores containing Tellurium, Arsenic, Antimony, Bismuth, etc.
I. Oxidised Ores—Acid.—Gold-bearing quartz may be taken as a typical example of this class of ores.
Oxidised Ores—Basic.—Ores containing a fair percentage of one or more of the following oxides, etc.—Limonite, Magnetite, Calcite, Dolomite, and Schistose or slaty substances.
II. Sulphide Ores (sometimes termed Pyritic ores or Sulphurets). These may contain one or more of the following sulphides (as well as traces of minerals found in Class III.), sulphides of iron, lead, copper, zinc, etc.
III. Ores of this class embrace those containing in appreciable quantity arsenic, antimony, bismuth compounds, tellurides of gold, silver, etc.; in brief, all ores coming under the assayer’s notice and not classified under groups I. and II.
Schemes for the examination of unknown ores prior to fire-assay have been given on preceding pages. These schemes serve as a guide to the student; the professional assayer can, from experience and his knowledge of mineralogy, generally determine by inspection the nature of an ore submitted for assay.
Having briefly considered the nature of the ores to be dealt with, the action of fluxes will now be discussed. The metallurgical student, in his study of the metals and the principles of metallurgy, will have received the ‘grounding’ necessary to the intelligent use of these reagents; he has now to apply his knowledge.
FLUXES
It will be seen that these are divided into two classes, Acid and Basic. A general rule may be laid down that an acid ore requires a basic flux and a basic ore an acid flux. That this is based on chemical principles will be seen on considering the nature of the complex silicates and borates occurring in the ‘slags’ formed by the fusion of ore and flux. Some ‘slags,’ especially those crystallized, have a definite chemical composition corresponding to a definite formula, whilst others are simply mixtures of different silicates. The following table gives the composition of the more important silicates.
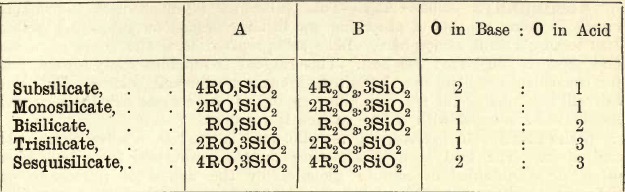
In column A the SiO2 is united with an oxide such as FeO- and in column B with an oxide such as Fe2O3.
A silicate containing one base is termed a Monobasic or Simple Silicate; two bases a Double Silicate, and so on. The fusibility of slags is dependent on the amounts of silica and bases they contain.
The subsilicates are very fusible, the monosilicates less so, and the bi- and tri- less so still; that is, as the proportion of base diminishes, the fusion point rises. Again, double silicates are more fusible than simple silicates. The mixture of two bases seems to behave similarly to a mixture of metals in an alloy—the melting point is lowered. The most fusible silicates are those of the alkalies, then those of lead, manganese, iron, copper, etc.; then those of the alkaline earths. For further information the student is referred to metallurgical treatises dealing with blast furnace charges.
The borates are another set of complicated compounds of some importance to the assayer. Some knowledge of the action of borax on bases has been obtained by the student in the course of blowpipe analysis. Borax acts as a flux for oxides of iron, lime, etc.
The chief acid flux, then, is silica, and in the column of basic fluxes will be found the names of a number of substances, the chief of which for the assayer’s purpose are the carbonates of sodium and potassium, litharge and fluor spar. These fluxes with silica form silicates of sodium, potassium, lead, and calcium, whilst the fluorine is of service in breaking up silicates. A mixture of K2CO3 and Na2CO3 alone or with other reagents is of much service in breaking up certain clays which are decomposed with difficulty when either carbonate is used alone. Litharge is a most powerful basic flux, besides being an ‘oxidiser’ and ‘collectant’ for gold and silver. As a ‘ flux ’ it combines with silica, forming silicates of lead. Also with certain ‘infusible’ oxides such as red oxide of copper, it is capable of uniting to form fusible compounds.
DESULPHURISERS AND SULPHURISERS
These terms explain themselves, the following equations showing the reactions :—
Desulphurisers—
PbS + Fe = FeS + Pb
PbS + KCN = KCNS + Pb
PbS + 2PbO = SO2 + 3Pb
Sulphurisers—
Fe + S = FeS
Fe + PbS = FeS + Pb
Sulphur acts as a reducer towards litharge, metallic lead being deposited and sulphur dioxide formed.
ABSORBENTS – Bone Ash. —This substance is much used in making cupels, it being capable of absorbing the litharge formed on subjecting molten lead to an oxidising atmosphere. Bone ash prepared from the bones of horses and sheep is considered the best. This reagent is obtained ready for use, and for convenience a little may be sifted through muslin and bottled. This fine ash will be found useful for sprinkling on top of the coarser ash in the cupel mould when a cupel with a fine surface is required.
SOLVENTS.—In fire-assaying metallic lead is used as a solvent for gold and silver. The lead is used sometimes in the granulated form, but more often it is obtained in minute globules by the action of a reducer on litharge. A shower of such globules on permeating the charge in a crucible picks up particles of gold and silver and collects them into a button at the bottom of the crucible.
COVER.—Salt alone or salt and borax may be used as covering agents. The salt on melting floats in a layer on the top of the charge, and prevents oxidation by excluding the air. A pinch of salt tends to prevent the too violent ebullition of a charge, the action here being somewhat similar to that of oil on water.
INQUARTATION.—Test Silver.—This will be required in the operation of Inquartation, to be described later on. To be suitable for this purpose the silver must contain no gold, and otherwise must be reasonably pure. A little copper will in many cases do no harm, thus allowing the use of silver coin ; but if pure test silver be required, it may be prepared as follows :—
A silver coin or piece of bullion is placed in a small beaker; add about 20 c.c. strong HNO3, gently heat in a fume chamber till all is in solution. Precipitate the silver as AgCl by slowly adding HCl until a slight excess is present. Boil for a few minutes, dilute with water, and filter. Wash the ppt. well with boiling water.
Test silver is frequently prepared from waste silver solutions. The silver in these is precipitated by adding salt (avoiding excess). The resulting AgCl is washed by decantation and then boiled in aqua regia to dissolve any gold present. Wash again and pure AgCl is obtained.
This AgCl may be treated in one of two ways — (a) by fusion with reducer and flux, or (b) reduction to Ag by Fe or Zn.
(a) By Fusion.—This method, though speedy, results in the loss of a certain amount of silver by volatilisation, etc.
The chloride is dried, weighed, and smelted in a small clay crucible (D), after mixing with the following charge—
AgCl……………………………………………………….1 part
Glass Powder………………………………………….1 part
Soda………………………………………………………1 part
Cover, a little borax and 8 or 10 grains of nitre.
Fuse in a hot fire; pour when tranquil into a hot clean mould. When cool, detach and clean the button from slag. If foil be desired, pass repeatedly through the rolls. If granulated, remelt and pour from a height of about 6 feet into a bucket of cold water.
(b) By Reduction.—Place the moist AgCl in a porcelain basin, add sheet zinc strips in the proportion of one part Zn to two parts Ag. Add a little H2SO4. Let stand over-night. Add more H2SO4 if any Zn is still undissolved. Wash the ‘cement’ silver well, dry and weigh. Fuse in a small plumbago crucible with a cover of charcoal powder, adding a little ‘borax glass’ and a small pinch of nitre. When melted, pour into a mould. Further treatment as in (a).
Before proceeding to the preparation of wet reagents, a short description will be given of the preparation of pure gold.
Proof Gold.—Weigh out about 100 grains ‘gold cornets’ (see Bullion Assaying). Place in a small flask (500 c.c.). Add gradually 50 c.c. aqua regia. Heat gently to boiling on a hot plate. When all the gold is dissolved, dilute with water to about 400 c.c. Stand a few hours; decant the solution from any precipitated AgCl into a large evaporating dish; evaporate till nearly all the acid is expelled and AuCl3 begins to separate out. Dilute to about 400 c.c. and filter into a glass flask (500 c.c.) through a double filter piper. Plug the neck of the flask with cotton-wool, stand for 4 to 6 days. Decant about seven-eighths of the solution through a filter paper; neglect the remainder for the present. Heat to boiling, and add an excess of a saturated solution of oxalic acid till the solution is colourless. Stand over-night. Pour off the solution and wash well with warm distilled water, then with ammonia water, then again with distilled water. Wash with dilute HCl and again with distilled water. Transfer the gold to a porcelain dish. Dry, fuse in a clean crucible in which some borax has been melted. Cover with borax glass and add a few grains of nitre. Pour into a clean waxed mould. When cool, detach the button from the slag, clean thoroughly, and pass through the rolls. The resulting gold should be about 999.95 fine.
WET REAGENTS USED IN FIRE-ASSAYING
Water.—This is used chiefly for washing ‘beads’ or ‘cornets’ of gold after inquartation and, as silver nitrate is present, the water used must be free from chlorine; therefore if tap-water be used it must give no reaction for chlorine. If chlorine be present the water must be distilled; and where a considerable quantity of distilled water is required, a self-filling copper still of one or two gallons capacity will be found convenient.
Nitric Acid for ‘Parting’. —This reagent is used for dissolving the silver from an alloy of silver and gold; and here again, as silver nitrate is formed, chlorine must be absent. Generally, nitric acid can be obtained free from chlorine, but if not, the chlorine may be precipitated by the addition of silver nitrate. A precipitate of silver chloride is obtained, from which, after standing for some days, the acid may be syphoned off.
For ordinary work the following solutions are made up :—
No, 1.—1 volume strong HNO3 + 2 volumes water (dist.).
No, 2.—1 volume strong HNO3 + 1 volumes water (dist.).
Where the bead consists largely of silver, No. 1 solution alone may be sufficient, but in many cases the No. 2 solution must also be used. A little silver nitrate may be added to these solutions as a precaution against chlorine. Nitrous acid may be present; and as this acid is a solvent for gold, any traces present must be driven off by boiling for a few minutes before use.
For bullion assays the Mint and Bank assayers generally make up their solutions as follows :—
No. 1.—S.G. 1.16 (21° Beaume)
No. 2.—S.G. 1.26 (32° Beaume)
For No. 1 solution, 8 parts HNO3, (S.G. 1.41; 44° Beaume) mixed with 15 parts distilled water make an acid of S.G. 1.16 (21° Beaume).
For No. 2 solution, 8 parts HNO3 (S.G. 1.41; 44° Beaume) mixed with 5 parts distilled water make an acid of S.G. 1.26 (32° Beaume).
The practice of many American assayers is to use for beads from ores only one acid of S.G. 1.20 (26° Beaume), and for bullion work the two solutions given above of S.Gs. 1.16 and 1.26.
Sodium Thiosulphate (Hypo) Na2S2O3,5H2O.—This salt is serviceable for the Russell process extraction tests for the solution of silver chloride.
Besides the reagents mentioned, the assayer will find it convenient to have beside him a set of the more common chemical reagents, including the strong acids and alkalies; also numerous materials such as oil, plumbago, etc. etc., which will be mentioned from time to time.
