Numerous manganiferous silver deposits occur throughout the southwestern United States which remain undeveloped due to their refractory nature. In some cases, silver (Ag) is entrapped within minerals such as pyrolusite (MnO2) and is not amenable to cyanide extraction. Similar deposits occur in which molybdenum (Mo) or copper (Cu) is entrapped within goethite (FeOOH) or similar minerals and is not amenable to conventional acid leach extraction.
Materials and Methods
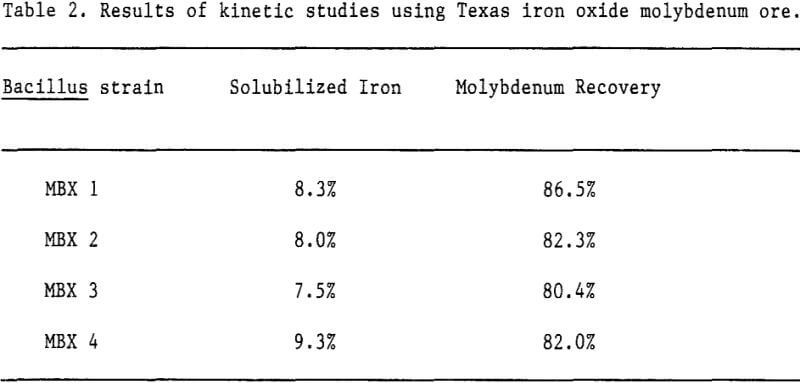
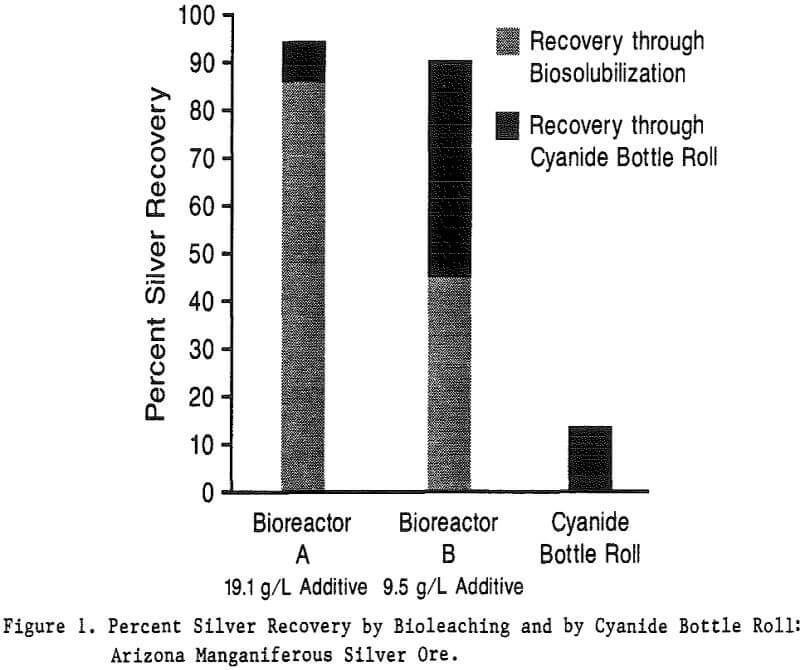
To compare rates of manganese and iron reduction by different strains of bacteria, Numerous small-scale (50-ml) kinetic studies were per-formed. To study manganese reduction, manganiferous silver ore samples (minus 200 mesh) were mixed with bacterial cultures in a mineral salts medium with and without a proprietary organic additive. The cultures were incubated on a rotator at room temperature. Fresh bacterial culture was added to the Arizona ore after 48 hours incubation. Solubilized manganese concentrations were monitored by atomic absorption (AA) during the studies while silver recoveries were determined by AA following cyanide extractions (5.0% cyanide, 24-hour roll).
After rates of bacterial manganese or iron and silver or moly recoveries, were compared through kinetic studies, the bacteria that most rapidly solubilized the desired metals were selected for continuous-stirred tank reactor (bioreactor) studies.
Results and Discussion
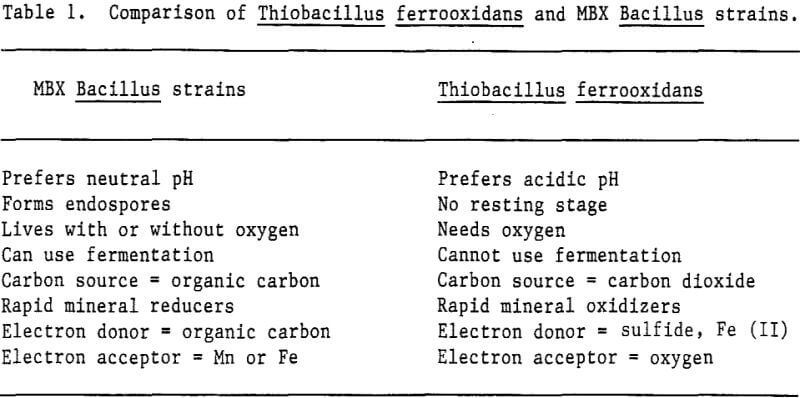
Over 400 bacterial isolates have been screened individually for their ability to reduce manganese and/or iron. As each new ore sample was screened for bioreductive microorganisms, the most promising isolates were lyophilized and frozen for further study. The best manganese and iron reducers isolated from the ore samples were all members of the bacterial genus Bacillus.
Because initial kinetic studies were carried out on a small scale, many different bacteria and parameters affecting these bacteria could be studied before scaling up. The primary purpose of the kinetics testing was to compare rates of mineral dissolution and resultant metal recovery by different strains of bacteria and combinations of bacteria.
The organic growth medium used at MBX is commercially affordable and simple to prepare (patent pending). This medium offers several advantages over the mineral salts medium. The bacteria grow more rapidly and the pH remains stable, never falling below 5.0.
Analyses of the heads, tails, and growth media from Bioreactors A and B showed that 100% of the copper was also biosolubilized into the organic growth medium. While the chemical mechanism for this is not yet certain, these results suggest that these bacteria may also be used to recover copper from refractory oxide ores.
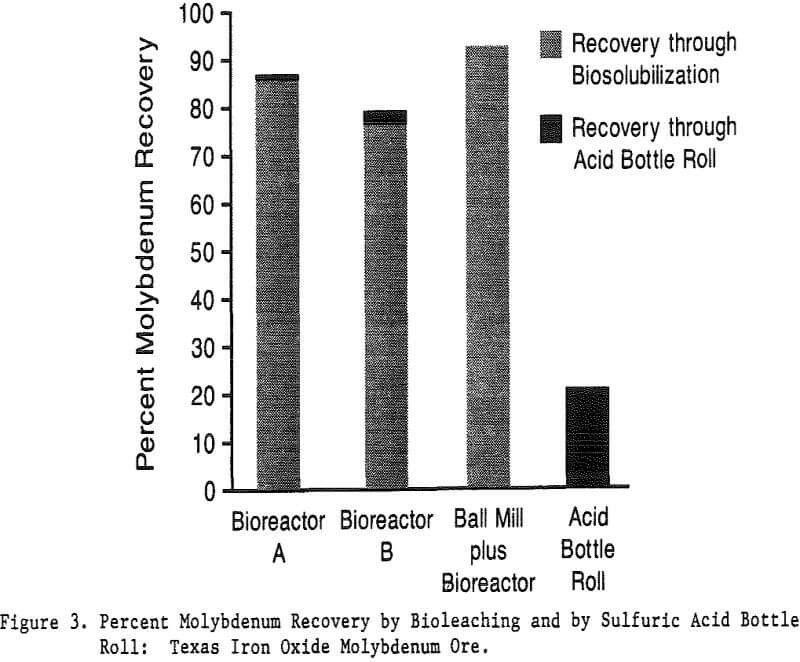
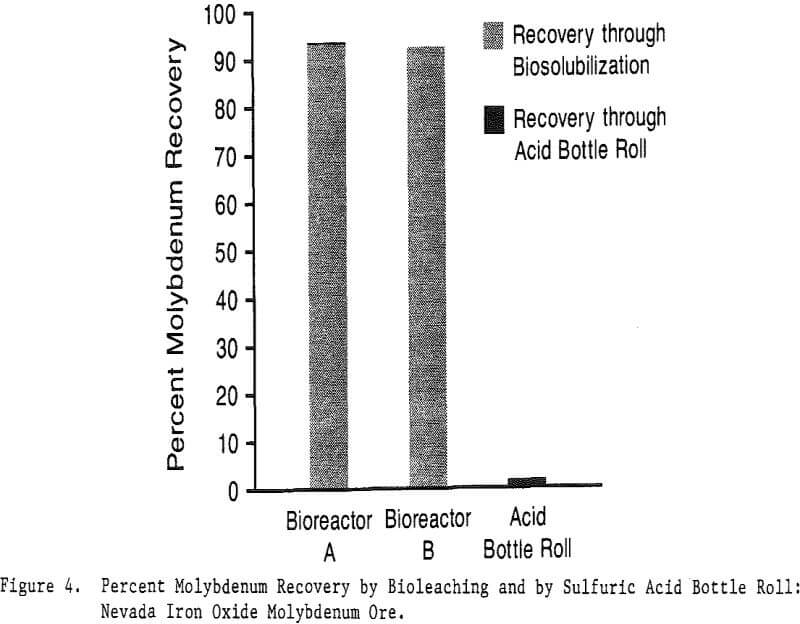
While laboratory results have been encouraging, many questions remain to be answered before scaling up to pilot plant tests. Scale-up factors have been favorable thus far. For instance, only 15% of the molybdenum was solubilized during 50-ml kinetic studies on the Nevada moly ore while 93.3% was solubilized during bioleaching in a 2-L bioreactor.
Fresh manganese/iron-reductive bacteria were often added to the bioreactors in the tests reported here. This was done to compensate for heavy metal toxicity in the growth medium. The use of more resistant bacterial isolates in future tests may reduce the number of bacterial additions needed.