The assistance that can be obtained from the microscope in solving ore-dressing problems has been increasingly appreciated in the recent years, as attested by the frequency of papers on the subject. Observations by means of binocular microscopes with magnifications up to 100 diameters and corresponding resolutions have long been common practice, and such observations are a great help in working out methods for the satisfactory treatment of an ore.
Further progress resulted in the use of metallographic equipment and magnifications up to 500 diameters. However, it is only recently that full use has been made of metallographic microscopes with useful magnifications up to 1000 diameters wherein particles 0.5 micron in diameter or less are clearly resolved. Such work requires skillful preparation of the specimen before the full capacity of the microscope can be made use of.
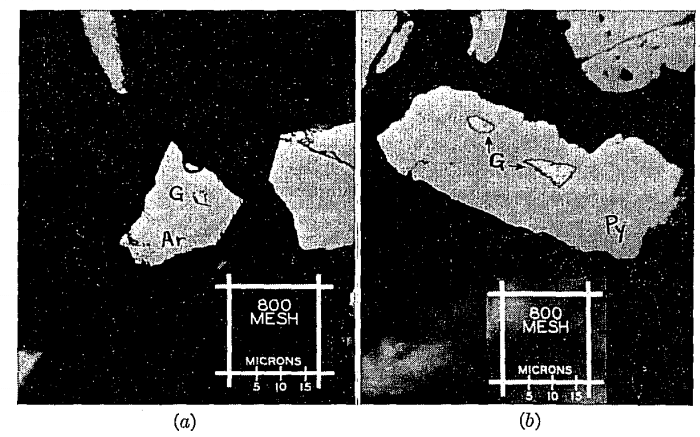
FIG. 2. Photomicrograph of a polished surface of a cyanidation residue reveals (a) an inclusion of gold (G) measuring approximately 2 by 3 microns, locked within a grain of arsenopyrite (Ar); (b) two minute gold particles (G) enclosed in a grain of pyrite (Py), the larger of the two inclusions being only about 5 microns in width. Magnification in each case = 1000X, with oil immersion objective.
The latter technique is of especial value in connection with cyanidation of gold and silver ores, because in certain ores some of the gold occurs as minute blebs or stringers in pyrite, the inclusions often being as fine as 1 micron in diameter. Two excellent examples of such occurrences published some years ago are (1) “Increasing Gold Recovery from Noranda’s Milling Ore,” by C. G. McLachlan, Trans. 112, A.I.M.E., 570, 1935, and (2) The Role of the Microscope in Ore Dressing, American Cyanamid Company, pp. 12-14, 1935.
Typical Gold Metallurgy Problems
A sample of auriferous-pyrite concentrate assaying 4.75 oz. per ton was submitted to our ore-dressing laboratory for the purpose of determining whether the gold could be extracted by means of straight cyanidation.
Preliminary tests on this sample indicated that this material was very refractory. Subsequent tests, in which the raw concentrate was reground to —325 mesh and cyanided for a long period of time with a strong solution, failed to improve the extraction materially. The lowest cyanide residue contained 0.33 oz. per ton. At this stage in the investigation it was decided to submit a sample of the above residue to the microscopical laboratory for the purpose of determining the form and manner of association of the gold. Accordingly, samples of the +325-mesh and —325-mesh residues were briquetted, polished, and examined with the metallograph at high magnification. It was noted that the gold occurred as metallic gold completely encased in pyrite and that the size of the gold particles was about 1 to 3 microns.
Inasmuch as minus 325 mesh is about the present economic limit for grinding, it was useless to proceed with further tests along the lines of finer grinding and cyanidation of the raw concentrate. Thus at an early stage in the investigation, the intelligent use of the microscope saved much useless work by narrowing down the lines of attack and pointing to a practical solution of the problem. In this case either roasting prior to cyanidation or direct smelting of the concentrate was definitely indicated.
For further information on the subject, the reader is referred to the latter paper, which gives an excellent description of a modern microscopic laboratory for such ore-dressing investigations, with practical examples, methods used, and bibliography.
An interesting paper by R. E. Head presented at the February, 1936, meeting of the A.I.M. and M.E., subject, “Physical Characteristics of Gold Lost in Tailings” indicates the possibilities of the microscope in the study of tailings losses. The following is an extract:
GOLD SAMPLE SURFACE CONTAMINATION
The experience gained by microscopic study of numerous gold ores and tailings has shown that there are pronounced differences in the physical characteristics of the minerals composing them, more especially of the gold. Repeated studies of tailings from flotation and cyanidation of gold ores has established the fact that surface contaminations on gold particles are often directly responsible for high gold losses; comparison of gold particles isolated from flotation concentrates and the resulting tailings have shown that the clean gold has been recovered and the tarnished or contaminated gold invariably lost in the tailing. Obviously, it is not possible to make such a comparison of gold ores treated by cyanidation, as the clean gold has been taken into solution; but when the gold found in cyanide tailing has a tarnished or coated surface, one may infer, with a reasonable degree of certainty, that the clean gold has been extracted. This premise is supported by experimental evidence obtained by isolating particles of tarnished gold and exposing them to cyanide solution in small parting cups. In one such experiment, tarnished gold particles picked from a cyanide tailing showed but slight evidence of dissolving at the end of 27 days. In this test, a cyanide solution of 1.6 lb. per ton was used, and the leach solution was decanted and renewed every 24 hr. The proof of cyanide attack was manifested by a noticeable thinning of the gold particles at the edges. A rim of a substance that appeared to be gelatinous was visible at the edges of the gold particles, and there is reason to believe that this material encased the entire surface of the gold particles. It is not known whether the gelatinous film is of secondary origin, resulting from a reaction between the cyanide and some substance or substances adhering to the gold surfaces in the form of a coating, or is an original constituent of the surface contamination which has been made visible through the dissolving of a small amount of gold at the margins of the particles.
