Free Cyanide vs Total Cyanide Determination

It is obviously of the highest importance in controlling the action of a solution to find out what strength of cyanide and alkali it contains at any given time. Under this head are the tests for free cyanide, “total” cyanide, hydrocyanic acid, and free or “protective” alkali. Besides these cyanide constituents, which have to be […]
Cyanide Reflux Distillation Apparatus
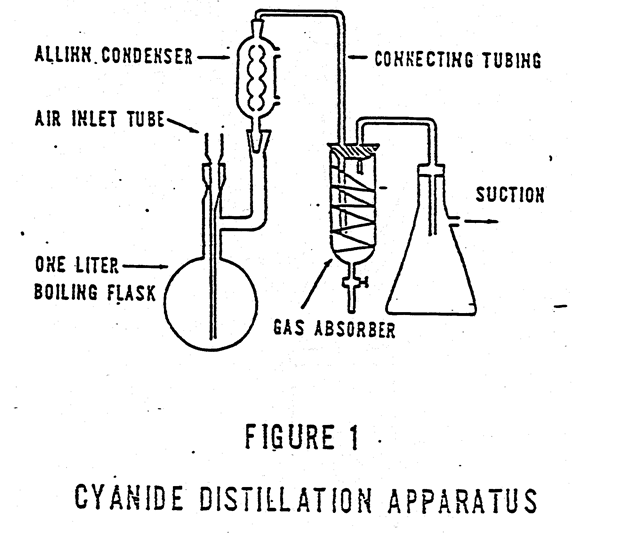
Distillation Procedure Samples without sulfide. Place 500 mL of the combined sample or an aliquot diluted to 500 mL in the 1 liter boiling flask. Pipet 50 mL of 1.25 N sodium hydroxide into the absorbing tube. If the apparatus in Figure 1 is used, add distilled water until the spiral is covered. Connect the […]
Thiocyanate Assay Determination
Determination of Thiocyanate by Colorimetric To a 100- cc Nessler tube add 50 cc water and 5 cc (more if necessary) of the cyanide solution to be tested, then add 2 cc HCl and 10 cc of 5 per cent solution of ferric chloride, FeCl3 Mix, and dilute to the 100-cc mark with water. If Prussian […]
Determination of Total Cyanide
Total cyanide is a term used to indicate, in terms of NaCN (or KCN), all the cyanogen existing in the form of simple cyanides, hydrocyanic acid, and the double cyanide of zinc. Procedure. Measure 25 cc of clear cyanide solution, add 10 cc of caustic soda-potassium iodide solution, and titrate with standard AgNO3 solution to […]
Free Cyanide Determination
You will perform this titration to obtain your Free Cyanide Determination. Take a 10ml aliquot of pregnant liquor. Make aliquot to 60ml with distilled water. Add 3 ml KI (10% solution) Add 5 ml of 1.5% NH4OH Titrate with 0.10 N Ag NO3 then; titre/100 = %CN Note: The endpoint is indicated by the first […]
Handling Waste Cyanide Solution
Tailings pulp carrying traces of cyanide and the discard of barren solutions in some cases constitutes a hazard to both humans and animals, and methods have been devised for destroying the contained cyanide. A recent paper “The Treatment of Cyanide Wastes”by Chlorination by J. G. Dobson published in the Sewage Works J., November, 1947, discusses […]
Gold Chlorination Processes & Methods
In Liddell’s Handbook of Nonferrous Metallurgy, Vol. 2, 1945, there is to be found a very complete account of the uses of chlorine as applied to the recovery of gold and silver, in Chlorination Processes & Methods. Introducing the chapter entitled “Chlorine Metallurgical Processes” Liddell says: Chlorine as a metallurgical agent appears to have lost ground […]
Bromocyanide Process
Bromo salts are a mixture of 57 per cent sodium bromide, NaBr, and 43 per cent sodium bromate, NaBrO3, in the form of light-gray, light- yellow, or reddish-brown crystals or powder. The use of bromo salts for treating a telluride concentrate in the Wright- Hargreaves plant at Kirkland Lake is described by J. T. Willey […]
Cyanide Regeneration Processes and Methods

Cyanide regeneration offers a practical means of overcoming the otherwise heavy cyanide consumption frequently encountered in the treatment of gold and, especially, silver ores, where cyanicides (cyanide consuming minerals) are present. Miscellaneous processes used for the extraction of gold and silver values by hydrometallurgical means are also discussed in this chapter. These include the carbon-cyanidation, […]
Carbon Cyanidation

Countercurrent carbon cyanidation with simultaneous dissolution of gold by cyanide and its adsorption by carbon offers several advantages over other carbon cyanidation processes as: the rate of dissolving the gold is faster, higher grade gold-bearing carbon is obtained, less carbon per ton of ore treated is required, separate dissolving and absorbing units are not needed, adsorption […]
