The purpose of the following research, as originally planned, was to investigate the influence of temperature upon the plate- amalgamation process. In order to consider the gold amalgamation process intelligently, it was first necessary to learn the nature of an amalgam. In the performance of this task it was found necessary to consult a large volume of literature and to perform experimental investigations. The conceptions of the nature of amalgams thus obtained have so important a bearing on the amalgamation-process as a whole, as well as upon the possible influences of temperature, that it was advisable to include them in the treatment of the subject. The broader title is therefore used, even though some features of the amalgamation process have been treated very briefly and others entirely omitted, there being nothing new to present regarding them and their development not required for a clear presentation of the matter in hand.
Gold Amalgamation Process History
Gold was one of the earliest metals known to man. Occurring in the metallic state, it could be picked up in nuggets or washed, as gold dust, from the sand and gravel of streams. Interesting descriptions of the processes employed in early times for the recovery of gold may be found in Pliny’s Natural History and in the treatise by Agricola on ores and minerals. But little progress in the treatment of the ores of gold was possible until efficient crushing machines were devised. This want was supplied by the introduction, about the beginning of the sixteenth century, of the stamp-mill to treat the ores of Saxony. Blanket-strakes, or similar devices, were used to catch the gold. Except in the gradual improvement of the machinery no important advances were made for many years. Hungary was the principal center of gold-milling in the eighteenth century. When the gold-fields of the southern United States were opened up, the processes used there were substantially the same as those employed in Hungary. But when the gold- fields of California were discovered, the great richness of the ores naturally stimulated inventive ability, and a legion of patent amalgamators resulted; these mostly survive at present only in the Patent Office records.
An improvement of lasting merit was the substitution of copper plates “amalgamated” or coated with a film of mercury, for the blanket-strakes, etc., until then employed to catch the gold liberated by crushing. This improvement is of such signal importance that it is to be regretted that the inventor is not known. The introduction of amalgamated plates into all the districts where gold-ores were being milled quickly followed, until at present their use is well-nigh universal for ores that are adapted to stamp-milling or dredging.
Amalgams
The amalgams have been a subject for research since the earliest days of the alchemists, and in few directions has so much work been done with such meager results in the acquiring of exact information. Research has proceeded along every practicable line; but it is not yet possible to say that the nature of amalgams is completely understood, although results obtained within the last few years have placed them in a much clearer light than ever before.
The assumption was early made that mercury forms an inter-metallic compound, or a series of compounds, with the metals with which it amalgamates. Many attempts have been made to isolate such compounds by the use of solvents, pressure, filtering, or volatilization, and many compounds have been described, although the most accurate work has shown in many cases that the results are not concordant enough to justify the assigning of definite formulae. The heat of formation of amalgams also has been studied by several investigators, most notably Berthelot. It was found that in some cases heat was evolved and in others absorbed. A simple and complete explanation of the results was not easy in most cases. The specific gravity and specific heat of amalgams have also received attention, the general conclusion being that amalgams are mixtures of a solid and a liquid. An investigation of the thermal expansion has shown that where the percentage of the metal other than mercury is very small the amalgam acts like a solution, but with greater percentage of the other metal (tin, lead, or zinc) it is apparently a mixture of a liquid and a solid.
The study of the electrical properties of amalgams has been more thorough because of their use in the preparation of standard cells. Investigation of the electrical conductivity has, in general, gone to show that, for small percentages of foreign metals in the mercury, the effect is that of a solution; while for greater percentages, apparently a mixture of liquid and solid is present; thus confirming the results of the investigations of the thermal expansion and specific gravity. The study of the electromotive force of amalgams has given more definite results. In the case of the alkali metals it has been demonstrated that more than one inter-metallic compound is formed with mercury. Haber concluded generally that the metals in amalgams either were in the atomic state or formed compounds of the type HgmM.
The microscopic examination of polished surfaces of amalgams has so far yielded but unsatisfactory results, due to the difficulties arising from the presence of the liquid phase. The study of the freezing-point curves has been more productive, but is not entirely concordant with the foregoing work. In the case of the tin-amalgams, Van Heteren has found that either tin, or a solid solution of mercury in tin, freezes out from the liquid down to -34.5° C., at which point a transformation takes place, and the new form, “probably mixed crystals,” freezes out to the solidification point of mercury. Roozeboom has shown that, in the series Cd-Hg, above 77 per cent, of Cd mixed crystals separate out; below 67 per cent, of Cd other mixed crystals of the Hg type separate out; between these ranges the action is more complex. Cd is insoluble in solid mercury.
The most complete study of amalgams by means of their cooling-curves has been done by Pushin, who carefully traced the cooling-curves of the series, Hg-Bi; Hg-Cd; Hg-Zn; Hg-Pb and Hg-Sn. These curves showing no evidence of the separation of inter-metallic compounds, the author concluded that the series Hg-Zn and Hg-Bi are mechanical mixtures, and the series Hg-Cd, Hg-Sn and Hg-Pb are solid solutions. These results can scarcely be accepted without comment, but criticism does not find a proper place here. Mercury forms the eutectic for all the series, except Hg-Zn, where the eutectic occurs at about 2½ per cent, of Zn. Tamman had already shown that for the series Hg-Zn and Hg-Bi the eutectic is very nearly pure mercury.
In conclusion, it may be said that with the alkali metals mercury forms a rather complicated series including several inter- metallic compounds. With some of the other metals the amalgams consist of solid or liquid solutions or mixtures of both. Others are not yet completely understood.
Gold and Silver Amalgams
- Native Amalgams.—Naturally occurring amalgams of gold and silver have been found in some localities. Schneider reports gold-amalgam occurring in small grains with the platinum of Colombia, and having approximately the composition Au4Hg5. An amalgam from the Mariposa region, California, analysed by Sonnenschein, was nearly Au3Hg4 in composition. Silver-amalgam is more common, and numerous occurrences and analyses are cited in Dana’s System of Mineralogy. The crystals of silver-amalgam are isometric, commonly dodecahedral, the analyses vary from Ag2Hg3 to nearly pure silver. Isometric crystals occurring in cavities filled with mercury, at Sala, Sweden, analysed by Sjogren, had the composition Ag2Hg3. But in the case of these amalgams it is only where there is excess of mercury present that we can be certain that no mercury has been lost by alteration. It is extremely difficult to free the amalgam from this excess of mercury; and the analyses do not seem to justify a more definite statement than that the composition of natural amalgams is somewhere near AuHg and AgHg.
- Artificial Amalgams.—Joule in 1850 described the preparation of a silver amalgam by the action of silver nitrate on mercury. He used a pressure of 72 tons (per square inch?) to press out the excess of mercury, and in this way produced a hard mass of shining crystals to which he gave the formula AgHg.
Henry treated finely-divided gold with mercury, removed the excess of the latter by pressing through chamois leather, and treated the residue with hot nitric acid, thus obtaining yellow shining crystals, to which he assigned the formula Au8Hg.
DeSousa attempted to obtain amalgams free from excess ot mercury, by holding them at a high temperature and ordinary pressure until there was no further loss of weight. The temperatures used were those of boiling mercury, sulphur and diphenylamine. At the temperature of boiling mercury, he obtained Au9Hg and Ag11Hg. In sulphur vapor Au9Hg and Au13Hg were produced, while with diphenylamine Au8Hg and Ag4Hg were found.
Merz and Weith later repeated this work more carefully, conducting the heating in a stream of hydrogen or nitrogen. They found that the loss of mercury was apparently continuous, though very slow, in the later stages, and concluded that the amalgams are decomposed at the boiling-point of mercury and that the small amount of mercury retained is mechanically held.
Knaffl, using HN03 of 1.35 sp. gr. at a temperature of 80° C., separated isometric crystals which he states to be nearly pure gold. Chester found that, beginning with cold nitric acid of 1.2 sp. gr., gently heating, and finishing with hot acid of 1.4 sp. gr., elongated needles could be obtained which appeared to be hexagonal prisms terminated with pyramids and base. These contained about 6 per cent, of mercury. Both Knaffl and Chester were investigating the crystallization of gold, and paid little attention to the mercury-content of the crystals. Wilm by the use of nitric acid obtained needles and prisms which contained from 9.87 to 11.45 per cent, of mercury. Fedorow states that the crystals left by the treatment of gold- amalgam with nitric acid are prismatic, belonging to the hexoctahedral class, being elongated in a direction perpendicular to the octahedron faces. Fremy states that a white crystalline mass having the composition AuHg4 can be separated from gold-amalgam. Many other statements in regard to the composition of gold-amalgams can be found scattered through technical journals and text books, but these are usually vague or are based on very slender evidence.
Kasantseff studied the action of nitric acid on solid and liquid gold-amalgams, concluding that the resulting alloys were not homogeneous in character. He also investigated the solubility of gold in mercury, by filtering a liquid amalgam through capillary tubes, and determined a solubility of 0.011 per cent; of gold at 0° C., 0.126 per cent, at 20° C. and 0.65 per cent, at 100° C. Dudley checked this work by allowing mercury containing 0.1 per cent, of gold to stand quietly for several months at 20° C. in a tall glass cylinder. The mercury decanted from the top at the end of this time contained 0.068 per cent, of gold, and after filtering through boxwood contained 0.06 per cent, of gold, indicating that the higher figures are due to the presence of fragments of gold or gold-amalgam so minute as to pass through the filtering device employed.
Berthelot studied the heat of formation of various silver amalgams; the results are significant, but are not easily explained. Ogg has investigated the chemical equilibrium between silver nitrate solution and mercury. He found that beyond a certain concentration of silver in the mercury a solid phase separates out. By the use of methods analgous to those generally adopted for hydrated salts the composition was determined to be Au3Hg4. This agrees very closely with the naturally occurring silver amalgam.
Research Work.—The work of Pushin on the cooling-curves of amalgams resulted in the acquiring of definite data in regard to their constitution. The attempt was therefore made to apply this method of study to the gold-amalgams. A nickel- copper thermo-electric couple was prepared and calibrated for the purpose; nickel-copper being employed rather than platin-rhodium because of its much greater sensitiveness, especially for the ranges of temperature within which it was intended to be employed.
The mercury used was chemically pure, having been purified by redistillation and treatment with acid. The gold employed was inquarted and parted with nitric acid, then dissolved in aqua regia, and precipitated by warming with ethyl alcohol, yielding a bright crystalline powder which was readily taken up by the mercury.
Since mercury forms the eutectic of the gold-mercury series, it is evident that, for mixtures high in mercury, the freezing- point curve coincides with the solubility curve of gold in mercury. The solubility of gold in mercury being known from the researches of Kasantseff and Dudley, it was easy to prepare an alloy of such composition that on cooling from the molten state the solid phase should begin to separate out near 100° C. This was placed in a small hard glass tube surrounded by a mixture of magnesia and asbestos, the whole being enclosed in a crucible and carefully covered to exclude drafts. It was raised to a temperature of 150° C. and slowly cooled. The cooling-curve was perfectly smooth, without any breaks whatever. The experiment was repeated several times with always the same result. Mixtures containing more gold were then employed and the initial heating was carried to higher temperatures, finally using a mixture in which it was evident that there was a solid phase still present near the boiling point of mercury. So much mercury is lost by volatilization at these high temperatures that any results which might have been obtained would have been untrustworthy.
No breaks in the cooling-curve could be detected, and it became evident that this method of research was unproductive of results. Probably this was because the lag of the solid phase was not marked enough to produce a distinct jog in the curve. It was not possible to work with mixtures high in gold, for the mercury is lost by volatilization below their fusing- points at ordinary pressure, and the facilities were not at hand for working under high pressures. The study of cooling-curves, therefore, had to be abandoned, without having secured any definite results.
But this work had shown that a solid phase is present at ordinary temperatures with alloys low in gold. The constitution of this solid phase was the next subject of investigation. It was inferred that if an alloy was prepared in which the gold was entirely in solution in the mercury, and this alloy subjected to the action of a solvent which would dissolve the mercury, but not attack the solid phase, then we would get practically the effect of the freezing-out of the solid phase by the removal of the mercury with which it was in equilibrium, finally leaving only the solid phase present.
Nitric acid was judged to be the most likely solvent to yield good results. An excess of the finely-divided gold was added to mercury, and the mixture held at 100° C. The resulting liquid was filtered off and treated with nitric acid of different strengths, both at 100° C. and at ordinary temperature. The results were very variable. Acid of 1.42 sp. gr. at 100° G. gave a brownish powder, of indistinct structure, which was nearly pure gold, becoming bright on ignition. With acid of less and less strength the solid contained more mercury, and with 1.1 acid the residue contained 13.62 atomic per cent, of mercury (i. e., 13.62 atoms of mercury in 100 atoms of the alloy). Numerous determinations on this residue showed it to be fairly uniform in composition throughout.
The residues were examined under the microscope by reflected light, using magnifications of 40 and 60 diameters. The powders from the stronger acid were indistinctly crystalline, apparently isometric where the form could be distinguished. The residue from the 1.1 acid was made up of distinct isometric crystals of a golden color, the octahedron and cubo-octahedron being the forms observed. Scattered throughout these

were a few long needles of a bright brassy yellow; their form could not be determined beyond that they were apparently elongated prismatic crystals. Good photographs of this crystal mixture could not be obtained because of the nature of the material, but Fig. 1 shows its general isometric character.
With the use of nitric acid of 1.05 sp. gr. different results were obtained. The entire residue consisted of the brassy yellow crystals, and these were now so well developed that they could be distinctly seen to be hexagonal prisms terminated with pyramids and the basal pinacoid. Their surfaces were bright, and they had evidently not been attacked by the acid. On analysis they proved to contain 17.44 atomic per cent, of mercury. They are shown in Fig. 2.
Subsequently to reaching these results it was discovered that A. H. Chester had described hexagonal crystals of gold-amalgam, stating that they contained about 6 per cent, of mercury. Fortunately Chester’s original material was still available for study, and upon examining it the pitted and corroded surface of the crystals was ample evidence that mercury must have been lost by the use of too strong acid. Chester used 1.2 acid at 18° C. at the start, finally finishing with strong hot acid, hence the composition obtained.
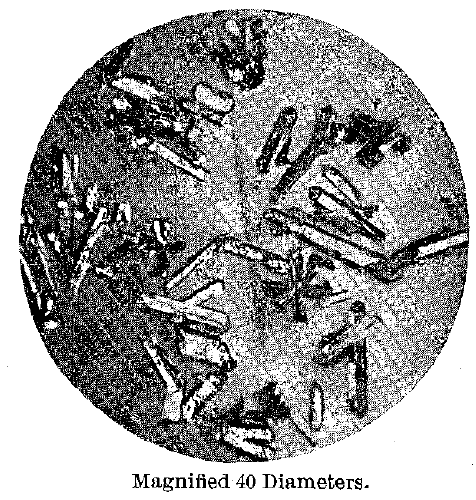
Fig.2 – Gold Mercury Series. Hexagonal Crystals, Inter-Metallic Compound or Solid Solutions
An attempt was made to study polished surfaces of the gold- mercury alloys. The surfaces were obtained by casting on glass and mica. In every case the excess of mercury present coated the crystals, giving only indistinct, rounded outlines from which nothing could be inferred.
Having thus learned the degree to which mercury is soluble in gold, the solubility of gold in mercury was next investigated. The differing results of Kasantseff and Dudley have already been cited. The methods used in checking these results were two in number: adding an excess of gold, heating to a higher temperature and filtering at the temperature desired; and suspending a thin sheet of gold below the surface of the mercury, holding the two for several days at the temperature at which it was desired to determine the solubility, and then analysing the mercury. In the former method fine cambric and close-grained chamois were used for the filters. It was found that the apparent solubility varied with the closeness of the filter, the pressure used in filtering, and also the degree to which the liquid had previously been heated, thus sustaining the contention of Dudley that the apparent solubility is due in part to the presence of minute solid particles which have passed through the filter. The results, though irregular, indicated that the solubility is at least not higher’than the figures given by Dudley, namely, 0.06 per cent, at 20° C. This conclusion was confirmed by the second method, which also gave a solubility of 0.25 per per cent, at 100° C. The first method gave, for the solubility at 100°, 0.35 per cent. In determining the solubility by diffusion, the liquid was held a few days at 100° C.; but the diffusion may perhaps not have completed itself, and the true solubility may be the mean of the two figures.
The solubility of gold in mercury is very low indeed. Judging from the direction of the curve, it is zero at the freezing- point of mercury, so that pure mercury is the eutectic of the gold-mercury series.
The results, though not entirely satisfactory, seem to justify the conclusion that in the gold-mercury series, when the content of gold is high, a solid solution of mercury in gold, which is isomorphous with gold, separates out. This solution may contain as much as 13 atomic per cent, of mercury. In the lower ranges of the series, what seems to be an intermetallic compound (holding gold or mercury in solid solution), containing 17.44 atomic per cent, of mercury, separates out: this crystallizes in the hexagonal system. From results on other amalgams, there remains a question as to whether this is really an intermetallic compound rather than a solid solution differing from the first. This question, and others not completely answered in the foregoing work, can be decided only after a large amount of very careful work. Their importance as bearing on the question actually under investigation did not appear to be great enough to justify undertaking such work at this time. Finally, gold is practically insoluble in solid mercury, and its solubility in liquid mercury for ordinary ranges of temperature is very slight indeed.
The investigation of the silver-mercury series presents all the difficulties of the gold-mercury series, with the added one, that no solvent could be found which would attack mercury without attacking the solid phase. The only results worth mentioning were obtained by placing a solution of silver in mercury on silver plates and allowing the silver plate to absorb the excess of mercury. In this way the crystals were obtained that are shown in Figs. 3 and 4. These show the presence of two series of crystals, one apparently isometric and the other prismatic

Fig. 3 – Silver Mercury Series. Isometric Crystals
with some modifying faces. From this evidence it may be inferred that silver is similar to gold in the forms which separate out from its series with mercury. The crystals were too small to analyse.
In this connection it must be remembered that Ogg has shown that silver forms with mercury the inter-metallic compound, Ag3Hg4.
What are the Mechanics of the Amalgamation Process
By the foregoing considerations the belief is confirmed that amalgamation is a physical rather than a chemical process. The solubility of gold in mercury is almost negligible, and the diffusion of the mercury into the gold, forming a solid solution or inter-metallic compound, is a rather slow process, unless the gold is very finely divided, whereas the “catching” of the gold by the mercury is almost instantaneous. It is from a study of the physical features of the process that information is to be derived regarding the proper conduct of the operation. Yet it must not be forgotten that although amalgamation is, with respect to any given particle of gold, an almost instantaneous process, with respect to the total mass of ore it is a continuous process, and any change, however slow, must in time produce either beneficial or detrimental effects. All the forces which operate must therefore be considered, and their relative effects determined.
With the exception of the tellurides, gold occurs in its ores as metallic or free gold, though frequently alloyed with greater or less amounts of silver, and, less frequently, with some other metals. Whether the tellurides of gold and silver are alloys or compounds is not a proper question for discussion here. The ores of gold are usually divided into two classes, “free milling” and “refractory” ores.
The closest practicable definition of these is that, in a “free- milling” ore, the gold occurs in such a form that it is readily and pretty completely recovered by crushing and amalgamation, while in refractory ores this is not the case. That the refractoriness of an ore may arise from one or more of a variety of conditions, will be easily seen after outlining the physical features of the amalgamation of a “free-milling” ore.
The purpose of the crushing operations, usually performed in a stamp-mill with water, is to free the gold from the adhering gangue. The action of a stamp has been aptly likened to that of a hammer cracking a nut and liberating the enclosed kernel. If the stamp has done its work properly, the pulp, as it passes through the battery-screens, consists of particles of gangue and particles of free gold. This pulp is caused to pass over copper plates coated with mercury in a thin film. No mineral matter other than metal is susceptible of being wetted by the mercury, and, in addition, all the ore-material other than the metal is much lower in specific gravity than mercury, and hence flows over the mercury surface without being affected by it. The gold, on the other hand, on coming in contact with the mercury is immediately wetted by it, just as any ordinary solid is wetted by water; the action of the two liquids, water and mercury, being identical from a physical standpoint, and their apparent differences due to the differences in their physical constants. This action is assisted by the fact that gold has a greater specific gravity than mercury, and therefore readily sinks beneath its surface; but this feature is not so important as earlier writers have claimed, for silver and the other lighter metals are readily amalgamated when their surfaces are in proper condition to be wetted by the mercury.
The gold particle, therefore, sinks beneath the surface of the mercury film which coats the plates, or, in mill-parlance, it is “caught.” If the diameter of the particle is smaller than the thickness of the mercury film, it produces no disturbing effect on the latter. If, however, its diameter be greater, as will always be the case with coarse gold, the condition of affairs produced is as shown in Fig. 5.
The mercury rises over the grain of gold. But, as is well known, every liquid has a tendency, due to surface-tension, to provide itself with a horizontal upper bounding-surface. In the attempt to do this in that part of the surface which is elevated by the gold grain lying beneath, the pull of the surface tension is resolved into components which press down the gold grain against the plate with a force which is considerable, compared with the dimensions and weights involved. This force begins to be exerted the instant the grain of gold is wetted by the mercury, and continues to operate until the grain is drawn beneath the surface; it constitutes the so-called “attraction” which mercury possesses for gold. Its exact amount may be computed, knowing the size and shape of the grain, the thickness of the film and the temperature. Under the influence of this force, especially if it is assisted by the splashing or dropping of the pulp, the grains of gold are caused to adhere to each other and to the plate with great tenacity.
A third factor, which is of great importance in causing amalgam to become hard, is a slow molecular flow, or some action of similar character. An amalgam of finely-divided gold and
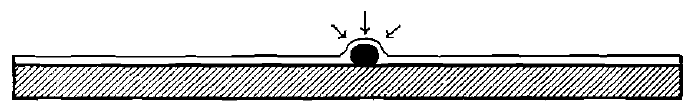
Fig.- 5 Effect of Contact of a Grain of Gold With a Thin Layer of Mercury
silver, made up to the consistency of a soft paste, will, after standing several days, become quite hard and rigid, but after kneading with the fingers assumes its original consistency. On standing again, it becomes as hard as before; and this operation may be repeated indefinitely. The hardening is much accelerated at higher temperatures, and, while it may be in part due to agglomeration caused by slight changes in the amount of mercury in solution in the silver or gold, resulting from the daily variations of temperature, yet chiefly it seems to be due to a movement of the particles of the metal, causing cohesion. Under the influence of these three factors the amalgam accumulated on a plate sometimes becomes quite hard, requiring considerable force to remove it.
It follows, accordingly, that any condition which tends to make the gold particles less easily wetted by the mercury has an important detrimental influence on amalgamation. Such conditions are many and varied. The influence of grease of any sort is so well known that it needs only to be mentioned. If the gold has not been liberated by the crashing, it cannot, of course, be caught; and if it has been only partially liberated, the tendency of the mercury to hold the gold particle may be overbalanced by the tendency of the current to sweep along the adhering particle of gangue. Gold alloyed or combined with other substances is frequently rendered by them incapable of being wetted by the mercury. This is the case with the tellurides. Some writers have asserted that gold occurs in certain ores as a sulphide, but the evidence adduced to prove such a contention is inconclusive. Gold alloyed with bismuth is similarly affected, and there may, perhaps, be other cases. Much more common, especially in ores which have been subject to alteration, are adherent coatings of minerals which are not wetted by the mercury. These are commonly oxides, but may be sulphides or other gangue-minerals. One case has been noted where the gold particles were coated with a film of chalcedonic silica. Such coatings are often difficult to remove, and it may be necessary to resort to some process other than amalgamation. A more lengthy discussion of them and their effects can be found in the references given below.
Another cause suggested for the refusal of mercury to wet gold at times is, that the latter may be in a strained state. Egleston reported that gold, after hammering or rolling until it was brittle, would not amalgamate, though it had been cleaned with acid. T. K. Rose reports that he was not able to confirm these observations. On rolling down pure gold until it was extremely brittle, I found that it would not amalgamate, even after cleaning with acid. But, on the surface being carefully cleaned with No. 0000 French emery-paper, it readily amalgamated demonstrating that the refusal of the mercury to wet its surface was due to the dust and dirt which had been pressed into it, and not to the strained condition of the metal.
Very fine gold may be carried along so rapidly by the current as not to come into contact with the mercury. To prevent this as far as possible, the outside plates are set at such a grade that as little water may be used as is consistent with securing free movement of the pulp. Even if brought into contact with the mercury, such gold may refuse to become wetted, because the mercury is not able to displace the air or liquid film already adhering to its fine particles. The operation of similar action in the case of some sulphides in the various flotation processes is already known. The refusal of mercury to wet very fine gold has been noted by many writers, and Louis has ascribed it to the existence of an allotropic modification of gold which is not wetted by mercury. This may be the case; but very finely-divided silver also refuses to be wetted by mercury, and it is perhaps better to leave the matter as an open question until further proof can be adduced.
It is usually the case that mercury is fed into the stamp-battery in order that, during the agitation of crushing, the gold may be brought into contact with the mercury, each grain being thereby thoroughly wetted. By the violent agitation during crushing, the mercury is subdivided into very small globules, thereby greatly increasing the chances that every gold particle will be brought into contact with the mercury and receive an adherent coating, which practically insures its being “caught” by the plates. This is for a triple reason: the weight of the particle is greatly increased; it is made more nearly spherical and correspondingly less likely to be carried off by the current of water; and, finally, the mercury film of the plates more readily “catches” a wetted particle than a dry one. To express the last statement in another way, mercury has less surface-tension against mercury than it has against gold. This subdivision of mercury into fine globules is one of the most important functions of the stamp-mill, and many authorities ascribe the excellence of stamp-milling as a process for the treatment of gold- ores principally to this feature of the operation.
But disadvantages are also introduced by this practice. The most important of these are the losses brought about by the “sickening” and “flouring” of the mercury. This arises chiefly from one or all of three causes. The first is purely mechanical. By the agitation in the battery the mercury is broken up into such small drops that the surface-tension of each is enormous, compared to its weight. This great surface-tension then keeps the globules perfectly spherical, and when they come into contact it is only at a point rather than along a line or upon a surface; the tendency of two globules to coalesce when they come into contact, or to sink beneath the mercury surface, is therefore very slight, and they are carried away with the pulp and lost. The tendency to settle is also very slight, just as a pebble, which falls to the earth rapidly, will, after being ground to dust, float in the air indefinitely.
The second cause is also mechanical, and arises from the presence of certain minerals which yield very fine slimes that adhere to the mercury surface, though not wetted by it. When covered with this fine coating, the globules can scarcely be made to coalesce with each other or with the mercury or the plates, and their loss is almost unavoidable, though the use of mercury-traps is of considerable assistance. An entirely similar action with water can be seen by sprinkling drops of water on a surface previously coated with lycopodium powder. Each drop becomes coated with the powder, taking on a spherical shape, if small, and moving about with the “quickness” of mercury. The drops can scarcely be made to coalesce, even if brought together with considerable force. The oxides of manganese and certain sulph-antimonides and sulph-arsenides are the worst offenders in this regard in amalgamation. It is reported that, in certain instances, the addition of various chemicals has been beneficial in preventing this action; but the data are not conclusive.
The third cause is chemical, and is a double source of loss. Soluble salts in the ore react with the mercury, causing it to be lost in solution, or precipitating other elements into it, causing it to become “sick.” “Sickness” is of two kinds. Any other metal alloyed with the mercury makes it much more viscous and less “quick.” A practical use is made of this fact in astronomical and physical work, where a mercury surface is used as a reflector. Mercury alone is too sensitive, vibrating constantly; but the addition of a small amount of tin causes it to stiffen enough to be perfectly satisfactory. In the case in hand this action is undesirable, for it causes the mercury to subdivide so readily that “flouring” takes place to an injurious degree. It is also undesirable for two other reasons: the fine slimes previously mentioned adhere much more readily to the surface of “sick ” mercury; and the metals in the mercury frequently reoxidize, forming a coating which almost prohibits the coalescing of the drops, or their being caught on the plates.
So far we have been concerned with actions that are comparatively rapid. But there are also others, of a slower rate, which remain to be considered.
The first is the absorption of the mercury by the other metals taking part in the process. It has already been pointed out that mercury is soluble in gold up to about 17.5 per cent. The rapidity of its absorption depends directly on the surface exposed by the gold, and account is taken of this fact in the usual statement of mill-men that more mercury is required to amalgamate fine gold than coarse. A large part of the mercury remaining in an amalgam after squeezing is held in solution in the gold, the remainder covering the particles in a film which assists in cementing the mass together.
Absorption by the Plates.—Important also is the absorption of the mercury by the plates. The investigation divides itself into two parts: the relative absorption of copper plates at different temperatures, and the relative absorption of different plates at ordinary temperature. For the former, sections of engravers’ plate were used. They had each an area of 4.15 sq. in. and a thickness of 0.067 in., weighing approximately 40 grams. They were thoroughly amalgamated (using cyanide solution and sand) and kept submerged in mercury, the one at 100° C. (boiling water) and the other at 0° C. (melting ice) for three weeks, the rate of absorption being determined by removing the plates at intervals, freeing from the excess of mercury by rubbing and then weighing. It was not, of course, possible to free the plates entirely from adhering mercury; but by the use of care in the rubbing it was found possible to obtain fairly concordant results.
The curves A and B of Fig. 6 show that, as might have been expected, the rate of absorption is much faster at the higher temperatures, and the total amount absorbed is greater. Evidently from the direction of the curve the absorption is not complete, even at the end of three weeks. In the case of the plate held at 100° C. another factor enters. Little gritty crystals are formed on the plate which are strongly adherent, though a few are removed by the rubbing. The plate, which was kept at 0° C., showed no indication of this, but in one instance a plate held at 20° C. did, to some extent. Apparently, then, the final limit of the absorption of mercury by copper plates is the formation of a solid solution or inter-metallic compound of a definite crystalline form.
The rate of absorption and total solubility depend on the temperature. One effect of the rise in temperature of plates that are in equilibrium can immediately be seen from an inspection of the diagram. On raising the temperature, more mercury will go into solution in the copper and also in the gold, and if more mercury is not fed to the battery, the plates will become dry and hard. The absorption is so slow that it will
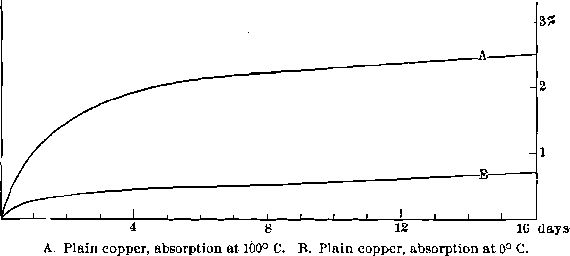
Fig.- 6 Absorption of Mercury by Copper Plate
require several weeks to complete itself at the raised temperature; and, during that time, progressively varying conditions will have to be dealt with. Hence the importance of keeping the temperature constant, to avoid changes in the hardness of the amalgam.
To obtain the relative absorption-rates of plain and silvered copper plates, two similar plates were cut from electrolytic sheet-copper, and ground down to plane surfaces and rounded edges. Each had an area of 4.85 sq. in. and a thickness of 0.06 in. One was then silver-plated to a thickness of 0.005 in. Both plates were then amalgamated, kept submerged in mercury at 20° C., and the rate of absorption determined as before. The silver plate retains a little more mercury as an adherent coat because of the greater specific gravity of the silver. The silver film somewhat restrains the diffusion of the mercury through it at first, but the ultimate effect differs little in the two cases.
The chief difficulty encountered in the use of plain copper plates is due to the fact that the small amount of copper which goes into solution in the mercury is very easily attacked by oxidizing agents and the salts which go into solution during the crushing of the ore, thus forming a tarnished film of copper salts on the surface of the mercury. Even the oxygen in solution in ordinary water produces this effect to an undesirable degree, but in the case of some ores containing certain
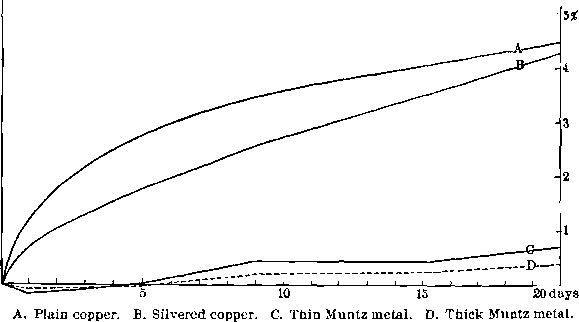
Fig. 7- Absorption of Mercury by Plates of Different Metals
soluble salts the action frequently is so strong that no gold can be caught, as very little clean mercury surface is exposed. What these salts are is not known, except in a few cases. The data are not accurate enough to base generalizations upon. This difficulty is obviated by silver-plating the copper plates. The silver is soluble in the mercury to a very much loss degree than is copper, and is also much less affected by the salts in question, probably not being at all acted on by the oxygen. The effect of the silver-plating on the absorption of the mercury by the copper is to restrain it at first, since the mercury has to diffuse through the silver. Eventually, however, the total amount absorbed is approximately the same, as is shown by the two curves, A and B, of Fig. 7.
The effect of the physical condition of the plates is also of great importance. It will have been noticed by comparing Figs. 6 and 7 that the rolled engraver’s plate at 100° C. absorbed more slowly than the electrolytic sheet-copper at 20° C. This must have been due to the difference in their structure, since the test was carefully repeated in each case.
How to Use Muntz Metal Plates
Muntz metal for battery- plates has been employed in Australia, and its use has been warmly commended, but apparently has not spread much in this country or elsewhere. The advantages claimed for it are that it is cheaper, lasts longer, requires less attention, is easier to remove the amalgam from, and does not discolor. The solubility of mercury in Muntz metals was investigated, and plates for this purpose were kindly prepared by Mr. Frederick Maeulen (to whom my grateful acknowledgements are made). The metal had the composition: zinc 38.1 and copper 61.9 per cent. Plate C had an area of 5.11 sq. in. with a thickness of 0.0256 in.; while plate D had an area of 4.92 sq. in. and a thickness of 0.014 in. The plates were annealed and kept submerged in an excess of mercury. The negative reading at the end of the 24 h. is probably due to the mercury dissolving out zinc faster than it was itself absorbed. The solubility of mercury in Muntz metal is seen to be very low. The divergence of the curves emphasises the fact that the rate of absorption is proportional to the surface exposed, plate C having a much larger surface in proportion to its weight. The percentages are of weight, referred to the original weight of the plate. The curves also indicate that the absorption is very slow, and is not completed within the time during which observations were taken.
Hockin and Taylor have shown that mercury dissolves the zinc out of brass. No copper could be detected in solution in the mercury at the end of the period. From these facts the reasons for the excellence of Muntz metal for plates become reasonably clear. Since no copper goes into solution in the mercury, no trouble is experienced from oxidizing agencies, and the necessity for silver-plating is removed in most cases. However, in working waste heaps (which probably contained copper sulphate) the Muntz metal plates coated badly; the small amount of zinc in the mercury producing even more undesirable results than would copper in such cases.
The Muntz metal consists almost entirely of an inter-metallic compound of copper and zinc. Mercury is very slightly soluble in this compound (perhaps not at all, for the slow gain in weight may be due to the slow replacement of one metal of the compound by the mercury). Gold is presumably also very slightly soluble in it; and this explains the slight adherence of the amalgam to these plates, for, beyond question, the strong adherence of amalgam to copper or silver plates is due to the slow diffusion of the gold into the metal. Roberts-Austen has shown that gold diffuses into lead when brought into contact with it; and it is well known that alloys can be produced by pressing together finely divided metals that are capable of forming an alloy. This slight adherence is both an advantage and a defect; for it allows a closer cleaning up of the amalgam, and also makes it more frequently necessary. The thickness of the mercury film on the plates must also be important. The thickness of the film which a plate of a given inclination will hold depends on the specific gravity of the holding-surface. Hence, silvered plates will hold a thicker film, and Muntz metal a thinner. Plates well coated with gold amalgam hold the thickest film possible. Hence, excessive scraping of plates is to be avoided, since the gain in daily yield of amalgam is more than counterbalanced by the loss of “catching” power.
Since the Muntz metal is an inter-metallic compound of zinc and copper, there could be no possibility of any galvanic action between the zinc and copper to affect the process. Muntz metal plates present many desirable features, but apparently the demand has not been great enough to lead to their manufacture.
How Amalgamation is Affected by Temperature
Whether variations of the temperature at which the process is conducted has any direct specific effect on amalgamation, has been the subject of much discussion. It will be impossible to refer here to all the statements made regarding this, but a few of the more significant must be mentioned. T. J. Grier states that when two batteries were run side by side on the same ore, the one at 50° F. and the other at from 60° to 70° F., considerably more gold was caught by the battery at the lower temperature. J. A. Church cites a case where water from old mine-workings was used that had a temperature of 135° F. at the battery; the plates coated badly and the recovery was very poor. T. A. Rickard says that at Black Hawk, Colo., the recovery is better in winter than in summer, and objects to the practice at the Britannia United, Ballarat, of warming the battery-water. Louis Janin says that no advantage is gained by heating the battery-water. At the Golden Star mill, Supt. Read changed a battery from 56° F. to 70° F. In 24 hr. the amalgam had nearly all scoured off. This was a silver plate; the copper was not affected. It is not stated whether all other conditions were kept constant, and it is hardly safe to comment on such an experiment unless all the conditions are known. J. A. Sanborn says that the objection to an increase in temperature is that the plates were prepared for a lower temperature, but that the adjustment may be made for any reasonable temperature. He also says that the chief objection to hot battery-water is the resultant increased solubility of deleterious salts. J. H. Thierman suggests that the heated water causes any grease which may be present to form more readily a film over the gold and mercury. A. von Dessauer found that the plate-amalgam contained from 20 to 35 per cent, of gold in the summertime, but only from 7 to 10 per cent, in winter. Besides these factors, R. H. Richards mentions two others, the attraction of mercury for gold and the cohesive power of the mercury. It should be noticed, however, that the only attraction mercury has for gold is the surface-tension pull, previously mentioned ; and that the facilitation of the coalescence of globules of mercury by a rise of temperature is not due to an increase of cohesive power, but to a decrease of their surface-tension.
The points in regard to the effect of temperature on the solubility of deleterious salts and resultant chemical reactions, their influence on “flouring,” and the influence of rise of temperature on the surface-tension and viscosity of the mercury, have already been treated as fully as seems necessary.
To determine whether other physical constants are appreciably affected by temperature-changes, two lines of investigation were followed. The purpose of the first was to learn whether the plasticity of the mixture of liquid and solid, ordinarily known as “amalgam,” varied to any sensible degree with ordinary ranges of temperature-change.
For this purpose an apparatus was designed and constructed, in which a pasty mass of amalgam was held in a cup that was rotated by a small electric motor. The cup was provided with sharp points on the inside, so that the amalgam was obliged to rotate with it. Submerged in the mass of amalgam was a stirrer, turning in a ball-bearing. The torsional pull on the stirrer, when the mass was rotated, was measured by a delicate helical spring. The whole apparatus was placed in a constant-temperature chamber, and numerous observations made over the range from 18° to 100° C. (60° to 212° F.). Both silver- and gold- amalgams of the consistency of thick cream were used; it was necessary to employ very finely divided metal in preparing the amalgam, in order to make it homogeneous. It was found that, as result of the increase of temperature, two conflicting changes tended to take place:—the amalgam tended to become harder from the increased absorption of the mercury by the gold, and softer from the decreased viscosity of the liquid mercury cementing the solid particles. The total change was very slight, and of the same order as the errors of observation due to the settling of the solid particles and the slight imperfections of the apparatus. A more detailed statement of the results, and a sketch of the apparatus, are therefore omitted. The investigation showed that the changes in plasticity induced by ordinary variations in temperature are negligible.
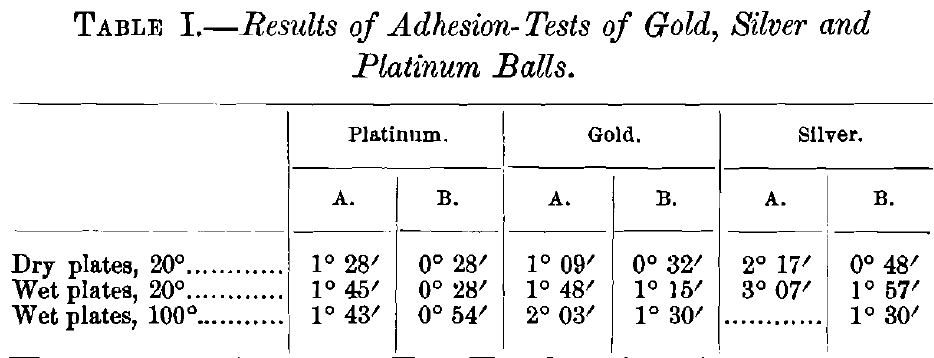
The purpose of the second investigation was to learn whether the adhesion of gold to mercury is sensibly affected by changes of temperature. To this end the inclination at which spheres of gold, silver and platinum, one-half an inch in diameter, will roll down an amalgamated copper-plate was observed. Two sets of angles were measured: that at which the spheres would start to roll from a state of rest (column A, Table I.), and the angle at which they would continue to roll after being started by a touch from a camel’s hair brush (column B, Table I.). The inclination was measured for the dry plate at ordinary temperature (20° C.), the amalgamated plate at the same temperature, and the amalgamated plate at 100° C. The gold and platinum spheres were perfect to within 0.001 in.; the silver sphere was not so perfect, and gave more irregular results. The angles recorded in Table I. are the average of several observations. The plate was somewhat roughened by the action of the mercury at the higher temperature; this is especially noticeable in the angles at which the balls would start themselves. The gold and silver balls were previously amalgamated for the determinations with the amalgamated plate, but the platinum ball, of course, was not. It is seen at once from an inspection of the results that the effect of variations of temperature on the adhesion of gold to mercury is also negligible.
These facts are so completely in accord with the data obtained by the metallurgical and chemical investigations, that there seems no escape from the conclusion that amalgamation of gold-ores is mainly, and above all, a simple mechanical process. Such chemical forces as enter into it are only slight, and are mainly detrimental, as previously outlined. The chief physical factor is the surface-tension of the mercury, which causes the mercury to cling in an adherent film to the plates employed, to wet the gold grains, and to draw them beneath its surface. It also acts detrimentally, tending to prevent the globules of mercury from coalescing again when they are very finely divided. The solution of the gold and silver by the mercury is very slight indeed; that of the copper is more noteworthy, and gives opportunity for undesirable chemical actions. The absorption of the mercury by gold, silver and copper is large in amount. With gold, what is probably an inter-metallic compound is formed with excess of mercury at 100° (whether it can form at ordinary temperatures is not certainly known); but this is of no great importance in the ordinary application of the process. Some sort of molecular flow causes the grains of the amalgam to adhere strongly together on standing, and the slow diffusion of the gold into the plate causes the amalgam to adhere strongly to copper and silvered plates. According to Richards, this diffusion does not extend to any great depth in the ordinary life of a plate.
The effect of changes in temperature on amalgamation is seen to be two-fold. The solubility of deleterious salts in the ore is, of course, increased by rise of temperature, their action is accelerated, and the solubility of copper in the mercury is increased, thus giving greater opportunity for these harmful reactions. But the most important effect is the disturbance of plate-equilibrium, resulting from changes of temperature. The thickness of the film of mercury which the plate will hold, and the absorption of mercury by the other metals concerned, are changed, and the mill-man then has to deal with varying conditions, a state of affairs to be avoided, if possible. From the physical standpoint, the temperature employed does not matter, so long as it is constant. From the chemical standpoint, a low temperature is better in most cases. But it should be mentioned, in passing, that both the viscosity and surface- tension of mercury are lessened by rise of temperature, the differences for ordinary ranges of temperature being slight. Under certain circumstances, where chemical action is negligible, it seems that it might be advisable to conduct the process at elevated temperatures, since the wetting of the grains by mercury is slightly facilitated by rise of temperature. The viscosity of mercury at 0° C. is 0.0169 dynes, and at 99°, 0.0123 dynes per sq. centimeter.
In the operation of the stamp-milling process the character and gold-content of the ore necessarily vary. If, in addition, the temperature, and with it all the foregoing factors, are allowed to vary, it becomes impossible to exert a proper control over the operation.
Amalgamation Explained
- Gold absorbs mercury, forming a solid solution which may contain as much as 13 atomic per cent, of mercury. Beyond this, an inter-metallic compound containing gold or mercury in solution (or a second solid solution) is formed, which contains 17.5 atomic per cent, of mercury. Ordinary amalgam, which is not in a state of equilibrium, consists of one or both of the foregoing, usually the former, mixed with an excess of mercury which coats the particles and causes them to cohere.
- Amalgamation is a physical process, the chemical actions involved being chiefly inimical (excepting those purposely induced). The gold grains are wetted by the mercury and sink beneath the surface of the mercury film on the plates; this is facilitated by feeding mercury to the stamp, so that the grains may be thoroughly wetted before coming in contact with the plates. The disadvantages of this procedure have already been discussed. The surface-tension of the mercury draws the gold beneath the surface, and holds it against the plate. By diffusion into the metal of the plates the amalgam often becomes strongly adherent. Silver-plating is useful, because it prevents the solution of the copper in the mercury, and, therefore, the harmful chemical reactions that result therefrom. Muntz metal plates exhibit the same effect, and, in addition, diffusion of amalgam into them is very slight, so that it is readily removed. Silvered plates will hold a thicker film of mercury than plain copper, and plates coated with gold amalgam a thicker film than either. This assists the “catching” of the gold.
- Variations in temperature make themselves felt in slight changes of a number of factors rather than large changes in any one. According to the relative importance of these factors in each case the total effect may vary. The most important undesirable effects of raising the temperature are the increased solubility of harmful salts, and a corresponding increase of the precipitation of base metals into the mercury; this both hinders its proper action and leads to its loss. Rise of temperature also diminishes the surface-tension and viscosity of the mercury, which allows it to be more readily “floured.” The force with which the gold is drawn beneath the mercury and held against the plate is also decreased. On the other hand, by an increase in temperature the wetting of the gold by the mercury and the “catching” of it by the plates is facilitated, as is the coalescing of the globules of mercury.
- Increase of temperature causes increased absorption of mercury by the gold and by the plates. Changes in temperature cause changes in all the foregoing factors. The retaining of a constant temperature is therefore most favorable to successful working. A comparatively low temperature is better where the influence of soluble salts in the ore has to be considered (which is usually the case); but when this may be neglected, as high a temperature as can economically be maintained, without variation, is most favorable to successful amalgamation.
In conclusion, I desire to express my indebtedness to Prof. H. S. Munroe, who directed the investigation; and also to Dr. Wm. Campbell, who kindly took the micro-photographs, and aided in the metallurgical inquiry.
The Amalgamation of Gold-Ores, BY THOMAS T. READ, NEW YORK, N. Y.
