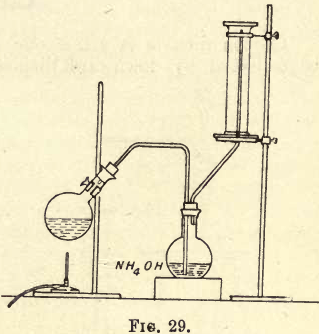
Ammonia Gas is so soluble in water that it cannot be collected as before over water in the pneumatic trough, and as it is lighter than air, fix up your apparatus as shown in fig. 29 and collect it by upward displacement.
Mix a little ammonium chloride with an equal amount of quicklime and put it in a test-tube and heat; you will smell ammonia coming off strongly. Repeat the experiment, only using sodium hydrate instead of quicklime; the same result follows. Moisten a piece of red litmus paper reddened by holding it over a little strong hydrochloric acid, and then hold it over the test-tube; it is turned blue.
Weigh out about 10 to 15 grams of ammonium chloride, powder it, and mix it with about an equal weight of powdered quicklime; transfer the mixture to your flask, and then cover the mixture with a little more quicklime in the flask ; fix in your cork and tubes as shown in fig. 29. Gently heat the flask; the ammonia gas soon comes off and passes through the second flask, containing a strong solution of ammonium hydrate, into the inverted jar. To tell when the jar is full, moisten a piece of turmeric paper and bring it near the mouth of the jar; if it turns brown it is full; place a cover over the mouth of the jar, and place another over the delivery tube. Collect three jars of the gas.
CaO + 2NH4Cl = CaCl2 + H2O + 2NH3
Ammonia Gas Laboratory Experiment I
Take a jar of the gas, invert it and bring a lighted taper into its mouth; a momentary flash of greenish-coloured flame is noticed near the taper, which is then extinguished.
Ammonia Gas Laboratory Experiment II
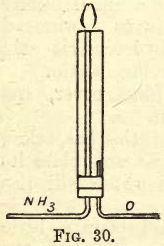
Pass your delivery tube up through a cork fitted into a large piece of glass tubing as shown in fig. 30. Also pass just through the cork a tube attached to an oxygen receiver. Start a current of oxygen through the tube, then a current of ammonia gas ; bring a light to top of the delivery tube of the ammonia gas, and you will find it will burn with a peculiar greenish-yellow flame.
Laboratory Ammonia Gas Experiment III
Pour 30 or 40 c.c. of strong ammonium hydrate into a small flat-bottomed flask; make a platinum spiral by winding a piece of platinum wire round a piece of small glass tubing, attach it to a small piece of tubing, then heat the spiral in a bunsen flame and plunge it into the flask containing the strong ammonium hydrate to within a centimetre or so of the solution; the spiral will continue to glow and the flask will become gradually filled with white fumes. The heated platinum causes the oxygen of the air to combine with the ammonia, forming nitrous acid (HNO2), which in turn acts upon the excess of ammonia and forms ammonium nitrite (NH4NO2), the white fumes you see in the flask.
Laboratory Experiment of Ammonia Gas IV
Put in a flask a few drops of concentrated hydrochloric acid and warm, then bring a jar of the gas inverted over the neck of the flask; dense white fumes immediately appear in the jar.
HCl + NH3 = NH4Cl
Laboratory Experiment with Ammonia Gas V
Take the remaining jar of the gas and bring it mouth downwards over the hole of the tray in a pneumatic trough filled with water; remove the cover-glass and you will see the water rising quickly in the jar, showing the solubility of ammonia gas in water.
NH3+ H2O = NH4OH
