Iron rusts or oxidises very readily, and, consequently, is rarely found in the metallic state in nature; such native iron as is found being generally of meteoric origin or imbedded in basalt and other igneous rocks. It chiefly occurs as oxide, as in magnetite, haematite, and in the brown iron ores and ochres. Chalybite, which is carbonate of iron, is an ore of great importance. Iron is found combined with sulphur in pyrrhotine and pyrites, and together with arsenic in mispickel. It is a common constituent of most rocks, imparting to them a green, black, or brown colour; and is present, either as an essential part or as an impurity, in most substances.
The chemistry of iron is somewhat complicated by the existence of two oxides, each of which gives rise to a well-marked series of compounds. Those derived from the lower oxide, known as ferrous salts, are generally pale and greenish. Ferric salts are derived from the higher oxide, and are generally red, brown, or yellow. The existence of these two well-marked families of salts renders the assay of iron comparatively easy, for the quantity of iron present in a solution can be readily measured by the amount of oxidising or reducing agent required to convert it from the one state into the other—that is, from ferrous to ferric, or from ferric to ferrous, as the case may be.
In the red and brown iron ores and ochres ferric iron is present; in chalybite the iron is in the ferrous state; and in magnetite it is present in both forms. Traces of iron in the ferrous state may be found (even in the presence of much ferric iron) by either of the following tests:—
- Ferricyanide of potassium gives a blue precipitate or green coloration; with ferric salts a brown colour only is produced.
- A solution of permanganate of potassium is decolorised by a ferrous salt, but not by a ferric one.
Traces of ferric iron can be detected (even in the presence of much ferrous iron) by the following tests:—
- By the brown or yellow colour of the solution, especially when hot.
- By giving a pink or red coloration with sulphocyanide of potassium.
Substances containing oxide of iron yield the whole of the iron as metal when fused at a high temperature with charcoal and suitable fluxes. The metal, however, will contain varying proportions of carbon and other impurities, and its weight can only afford a rough knowledge of the proportion of the metal in the ore. There are two or three methods of dry assay for iron, but they are not only inexact, but more troublesome than the wet methods, and need not be further considered. Chalybite and the hydrated oxides dissolve very readily in hydrochloric acid; haematite and magnetite dissolve with rather more difficulty. Iron itself, when soft, is easily soluble in dilute hydrochloric, or sulphuric, acid. Pyrites, mispickel, &c., are insoluble in hydrochloric acid, but they are readily attacked by nitric acid. Certain minerals, such as chrome iron ore, titaniferous iron ore, and some silicates containing iron, remain in the residue insoluble in acids. Some of these yield their iron when attacked with strong sulphuric acid, or when fused with the acid sulphate of potash. Generally, however, it is better in such stubborn cases to fuse with carbonate of soda, and then attack the “ melt ” with hydrochloric acid.
When nitric acid, or the fusion method, has been used, the metal will be in solution in the ferric state, no matter in what condition it existed in the ore. But with dilute hydrochloric or sulphuric acid it will retain its former degree of oxidation. Hydrochloric acid, for example, with chalybite (ferrous carbonate) will give a solution of ferrous chloride; with haematite (ferric oxide) it will yield ferric chloride; and with magnetite (ferrous and ferric oxides) a mixture of ferrous and ferric chlorides. Metallic iron yields solutions of ferrous salts. It is convenient to speak of the iron in a ferrous salt as ferrous iron, and when in the ferric state as ferric iron. Frequently it is required to determine how much of the iron exists in an ore in each condition. In such cases it is necessary to keep off the air whilst dissolving; the operation should, therefore, be performed in an atmosphere of carbonic acid.
Separating iron from other substances
The separation of the iron from the other substances is as follows:—Silica is removed by evaporating the acid solution, and taking up with acid, as described under Silica; the whole of the iron will be in solution. The metals of Groups I. and II. are removed by passing sulphuretted hydrogen, and at the same time the iron will be reduced to the ferrous state. The solution should be filtered into a 16 oz. flask, boiled to get rid of the gas, and treated (whilst boiling) with a few drops of nitric acid, in order to convert the whole of the iron into the ferric state. When this condition is arrived at, an additional drop of nitric acid causes no dark coloration. The boiling must be continued to remove nitrous fumes. Next add caustic soda solution until the colour of the solution changes from yellow to red. The solution must be free from a precipitate; if the soda be incautiously added a permanent precipitate will be formed, in which case it must be redissolved with hydrochloric acid, and soda again, but more cautiously, added. After cooling, a solution of sodium acetate is added until the colour of the solution is no longer darkened. The solution, diluted to two-thirds of the flaskful with water, is heated to boiling. Long-continued boiling must be avoided. The precipitate is filtered quickly through a large filter, and washed with hot water containing a little acetate of soda.
The precipitate will contain all the iron and may also contain alumina, chromium, titanium, as well as phosphoric, and, perhaps, arsenic acids.
Dissolve the precipitate off the filter with dilute sulphuric acid, avoiding excess, add tartaric acid and then ammonia in excess. Pass sulphuretted hydrogen, warm, and allow the precipitate to settle. Filter and wash with water containing a little ammonic sulphide.
GRAVIMETRIC METHOD
Dissolve the precipitate in dilute hydrochloric acid ; peroxidise with a few drops of nitric acid and boil, dilute to about 200 c.c., add ammonia (with constant stirring) till the liquid smells of it, and heat to boiling. Wash as much as possible by decantation with hot water. Transfer to the filter, and wash till the filtrate gives no indication of soluble salts coming through. The filtrate must be colourless and clear. The wet precipitate is very bulky, of a dark-brown colour and readily soluble in dilute acids, but insoluble in ammonia and dilute alkalies. When thrown down from a solution containing other metals it is very apt to carry portions of these with it, even when they are by themselves very soluble in ammoniacal solutions. It must be dried and ignited, the filter paper being burnt separately and its ash added. When further ignition ceases to cause a loss of weight, the residue is ferric oxide (Fe2O3), which contains 70 per cent, of iron. The weight of iron therefore can be calculated by multiplying the weight of oxide obtained by 0.7.
The presence of ammonic chloride causes loss of iron during the ignition, and organic matter causes an apparent loss by reducing the iron to a lower state of oxidation. When the iron in the solution much exceeds 0.2 gram the volumetric determination is generally adopted, as the bulkiness of the precipitate of ferric hydrate makes the gravimetric method very inconvenient.
VOLUMETRIC METHODS
As already explained these are based on the measurement of the volume of a reagent required to bring the whole of the iron from the ferrous to the ferric state (oxidation), or from the ferric to the ferrous (reduction). Ferrous compounds are converted into ferric by the action of an oxidising agent in the presence of an acid. Either permanganate or bichromate of potash is generally used for this purpose.
Ferric compounds are reduced to ferrous by the action of:
- Stannous chloride;
- Sulphuretted hydrogen;
- Sodium sulphite ; or
- Zinc.
The processes, then, may be divided into two kinds, one based on oxidation and the other on reduction. In each case the titration must be preceded by an exact preparation of the solution to be assayed in order that the iron may be in the right state of oxidation.
PERMANGANATE & BICHROMATE METHODS
These consist of three operations:
- Solution of the ore;
- Reduction of the iron to the ferrous state; and
- Titration.
Solution
The only point to be noticed concerning the first operation (in addition to those already mentioned) is that nitric acid must be absent. If nitric acid has been used, evaporate to dryness, of course without previous dilution; add hydrochloric or sulphuric acid, and boil for five or ten minutes. Dilute with water to about 100 c.c., and warm until solution is complete.
The reduction is performed by either of the following methods:
- 10FeS04+2KMn04 + 8H2SO4=5Fe2(SO4)3 + 2MnSO4 + K2SO4 + 8H2O.
- 6FeCl2 + K2Cr2O7+ 14HCl=3Fe2Cl5+ Cr2Cl6 + 2KCl + 7H2O.
- Fe2Cl6 +SnCl2=2FeCl2+SnCl4.
- Fe2Cl6+SH2=2FeCl2+2HCl + S.
- Fe2Cl6+Na2SO3+H2O = 2FeCl2+Na2SO4+2HCl
- Fe2Cl6+Zn=2FeCl2 + ZnCl2
- With Stannous Chloride.—Fill a burette with a solution of stannous chloride, and cautiously run the liquid into the hot assay solution (in which the iron is present as chloride) until the colour is discharged. A large excess of the stannous chloride must be avoided. Then add 5 c.c. of a 2½ per cent, solution of mercuric chloride, this will cause a white precipitate (or a grey one if too large an excess of the stannous chloride has been added). Boil till the solution clears, cool, dilute, and titrate.
- With Sulphuretted Hydrogen.—Cool the solution and pass through it a current of washed sulphuretted hydrogen till the liquid smells strongly of the gas after withdrawal and shaking. A white precipitate of sulphur will be formed, this will not interfere with the subsequent titration provided it is precipitated in the cold. If, however, the precipitate is coloured (showing the presence of the second group metals), or if the precipitation has been carried out in a hot solution, it should be filtered off. Boil the solution until the sulphuretted hydrogen is driven off; this may be tested by holding a strip of filter paper dipped in lead acetate solution in the steam issuing from the flask. The presence of sulphuretted hydrogen should be looked for rather than its absence. It is well to continue the boiling for a few minutes after the gas has been driven off. Cool and titrate.
- With Sodium Sulphite.—Add ammonia (a few drops at a time) until the precipitate first formed redissolves with difficulty. If a permanent precipitate is formed, redissolve with a few drops of acid. To the warm solution add from 2 to 3 grams of sodium sulphite crystals. The solution will become strongly coloured, but the colour will fade away on standing for a few minutes in a warm place. When the colour is quite removed, add 20 c.c. of dilute sulphuric acid, and boil until the steam is quite free from the odour of sulphurous acid. Cool and titrate.
- With Zinc.—Add about 10 grams of granulated zinc; if the hydrogen comes off violently add water; if, on the other hand, the action is very slow, add sufficient dilute sulphuric acid to keep up a brisk effervescence. The reduction is hastened by warming, and is complete when the solution is quite colourless and a drop of the liquid tested with sulphocyanate of potassium gives no reaction for ferric iron. Filter through “ glass wool ” or quick filtering paper. The zinc should be still giving off gas rapidly, indicating a freely acid solution; if not, acid must be added. Wash with water rendered acid. Cool and titrate.
With regard to the relative advantages of the different methods they may be roughly summed up as follows:—The stannous chloride method has the advantage of immediately reducing the ferric iron whether in hot or cold solution and under varied conditions in regard to acidity, but has the disadvantage of similarly reducing salts of copper and antimony, which, in a subsequent titration, count as iron. Moreover, there is no convenient method of eliminating any large excess of the reagent that may have been used; and, consequently, it either leaves too much to the judgment of the operator, or entails as much care as a titration. Students generally get good results by this method.
The sulphuretted hydrogen method also has the advantage of quick reduction under varying conditions, and the further one of adding nothing objectionable to the solution ; in fact it removes certain impurities. The disadvantages are the necessity for boiling off the excess of the gas, and of filtering off the precipitated sulphur, although this last is not necessary if precipitated cold. The tendency with students is to get high results. The sodium sulphite method has the advantages of being clean and neat, and of requiring no filtration. On the other hand it requires practice in obtaining the best conditions for complete reduction; and, as with sulphuretted hydrogen, there is the necessity for boiling off the gas, while there is no simple and delicate test for the residual sulphurous acid. In addition, if an excess of sodium sulphite has been used and enough acid not subsequently added, the excess will count as iron. Students generally get low results by this method.
The advantages of the zinc method are, that it is easily worked and that the excess of zinc is readily removed by simply filtering. The disadvantages are the slowness with which the last portions of ferric iron are reduced, the danger of loss by effervescence, the precipitation of basic salts, and, perhaps, of iron, and the loading of the solution with salts of zinc, which in the titration with bichromate have a prejudicial effect. The tendency in the hands of students is to get variable results, sometimes low and sometimes high.
Generally speaking, the sulphuretted hydrogen and sodium sulphite methods are to be preferred. Carefully worked each method will yield good results.
The titration may be done with a standard solution of (1) permanganate of potash, or (2) bichromate of potash.
- With Permanganate of Potash.—Prepare a standard solution by dissolving 2.82 grams of the salt and diluting to one litre. The strength of this should be 100 c.c. =0.5 gram of iron, but it varies slightly, and should be determined (and afterwards checked every two or three weeks) by weighing up 0.2 gram of iron wire, dissolving in 10 c.c. of dilute sulphuric acid, diluting to about 100 c.c., and titrating.
The standard solution must be put in a burette with a glass stopcock, as it attacks india-rubber. The assay should be contained in a pint flask, and be cooled before titrating. The standard solution must be run in until a pinkish tinge permeates the whole solution; this must be taken as the finishing point. When certain interfering bodies are present this colour quickly fades, but the fading must be ignored. With pure solutions the colour is fairly permanent, and a single drop of the potassium permanganate solution is sufficient to determine the finishing point.
- With Bichromate of Potash.—Prepare a standard solution by dissolving 4.39 grams of the powdered and dried salt in water, and diluting to 1 litre. This solution is permanent, its strength is determined by dissolving 0.2 gram of iron wire in 10 c.c. of dilute sulphuric acid, diluting to about a quarter of a litre, and titrating.
Also prepare a test solution by dissolving 0.1 gram of ferricyanide of potassium in 100 c.c. of water. This solution does not keep well and must be freshly prepared.
An ordinary burette is used. The assay is best contained in a glazed earthenware dish, and may be titrated hot or cold. To determine the finishing point, place a series of drops of the ferricyanide solution on a dry white glazed plate. The drops should be of about the same size and be placed in lines at fairly equal distances. The bichromate is run in, in a steady stream, the assay solution being continuously stirred until the reaction is sensibly slackened. Then bring a drop of the assay with the stirrer in contact with one of the test drops on the plate. The standard can be safely run in 1 c.c. at a time, so long as the test drop shows signs of a precipitate. When only a coloration is produced run in cautiously a few drops at a time so long as two drops of the assay gives with the test a colour which is even faintly greener than two drops of the assay solution placed alongside. The finishing point is decided and practically permanent, although it demands a little practice to recognise it. The titration with permanganate of potassium has the advantage of a more distinct finishing point and easier mode of working; its application, however, is somewhat limited by the disturbing effects of hydrochloric acid. The bichromate method has the advantage of a standard solution which does not alter in strength, and the further one of being but little affected by altering conditions of assay. Hydrochloric acid has practically no effect on it. Both methods give accurate results and are good examples of volumetric methods.
The following results illustrate the extent to which the methods may be relied on; and the influence which the various conditions of experiment have on the assay.
Solutions of ferrous sulphate and of ferrous chloride were made containing 0.5 gram of iron in each 100 c.c., thus corresponding to the standard solutions of permanganate and bichromate of potassium. These last were prepared in the way already described. The solution of ferrous sulphate was made by dissolving 5.01 grams of iron wire in 100 c.c. of dilute sulphuric acid and diluting to 1 litre. A similar solution may be made by dissolving 24.82 grams of pure ferrous sulphate crystals in water, adding 100 c.c. of dilute sulphuric acid, and diluting to 1 litre.
Rate of Oxidation by Exposure to Air
This is an important consideration, and if the rate were at all rapid would have a serious influence on the manner of working, since exclusion of air in the various operations would be troublesome. 20 c.c. of the solution of ferrous sulphate were taken in each experiment, acidified with 10 c.c. of dilute sulphuric acid, and diluted to 100 c.c. The solution was exposed, cold, in an open beaker for varying lengths of time, and titrated with permanganate of potassium.
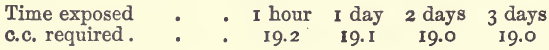
These results show that the atmospheric oxidation in cold solutions is unimportant. With boiling solutions the results are somewhat different; a solution which at the outset required 20 c.c. of permanganate of potassium, after boiling for an hour in an open beaker (without any precautions to prevent oxidation), water being added from time to time to replace that lost by evaporation, required 19.2 c.c. If the solution be evaporated to dryness the oxidising power of concentrated sulphuric acid comes into play, so that very little ferrous iron will be left. A solution evaporated in this way required only 2.2 c.c. of permanganate of potassium.
Effect of Varying Temperature
In these experiments the bulk was in each case 100 c.c., and 10 c.c. of dilute sulphuric acid were present. The permanganate required by
1 c.c. of ferrous sulphate was, at 15° 1.0 c.c., and at 70° 1.1 c.c.
10 c.c. of ferrous sulphate was, at 15° 9.7 c.c., and at 70° 9.8 c.c.
100 c.c. of ferrous sulphate was, at 15° 97.7 c.c., and at 70° 96.8 c.c.
The lower result with the 100 c.c. may be due to oxidation from exposure.
Effect of Varying Bulk
The following experiments show that considerable variations in bulk have no practical effect. In each case 20 c.c. of ferrous sulphate solution and 10 c.c. of dilute acid were used.
![]()
Effect of Free Sulphuric Acid
Free acid is necessary for these assays; if there is an insufficiency, the assay solution, instead of immediately decolorising the permanganate, assumes a brown colour. The addition of 10 c.c. of dilute sulphuric acid suffices to meet requirements and keep the assay clear throughout. The following experiments show that a considerable excess of acid may be used without in the least affecting the results. In each case 20 c.c. of ferrous sulphate were used.
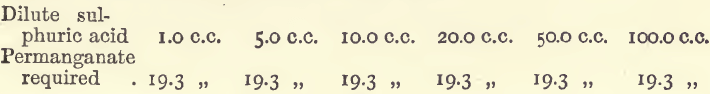
Effect of Foreign Salts
When the assay has been reduced with zinc varying quantities of salts of this metal pass into solution, the amount depending on the quantity of acid and iron present. Salts of sodium or ammonium may similarly be introduced. It is essential to know by experiment that these salts do not exert any effect on the titration. The following series of experiments show that as much as 50 grams of zinc sulphate may be present without interfering.
![]()
Magnesium, sodium, and ammonium salts, are equally without effect.

Effect of Varying Amounts of Iron
It is important to know within what limits the quantity of iron in an assay may safely vary from that used in standardising. In the following experiments the conditions as to bulk, acidity, and mode of working were the same as before:—
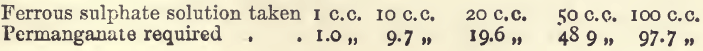
The ferrous sulphate solution is here a little weaker than that of the permanganate of potassium, but the results show that the permanganate required is proportional to the iron present.
Titrations in Hydrochloric Solutions
These are less satisfactory than those in sulphuric solutions, since an excess of hydrochloric acid decomposes permanganate of potassium, evolving chlorine, and since the finishing point is indicated, not by the persistence of the pink colour of the permanganate, but by a brown coloration probably due to perchloride of manganese. Nevertheless, if the solution contains only from 5 to 10 per cent, of free hydrochloric acid (sp. g. 1.16) the results are the same as those obtained in a sulphuric acid solution. Equal weights (0.1 gram) of the same iron wire required exactly the same quantity of the permanganate of potassium solution (20 c.c.) whether the iron was dissolved in dilute sulphuric or dilute hydrochloric acid. The following series of experiments are on the same plan as those given above with sulphuric acid solutions. A solution of ferrous chloride was made by dissolving 5.01 grams of iron wire in 50 c.c. of dilute hydrochloric acid and diluting to 1 litre. The dilute hydrochloric acid was made by mixing equal volumes of the acid (sp. g. 1.16) and water.
Rate of Atmospheric Oxidation
20 c.c. of the ferrous chloride solution were acidified with 10 c.c. of the dilute hydrochloric acid and diluted to 100 c.c. This solution was exposed cold in open beakers.
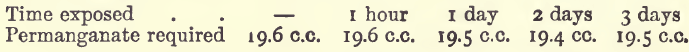
Similar solutions boiled required, before boiling, 20 c.c.; after boiling for one hour, replacing the water as it evaporated, 19.3 c.c.; and after evaporation to a paste and redissolving, 17.0 c.c.
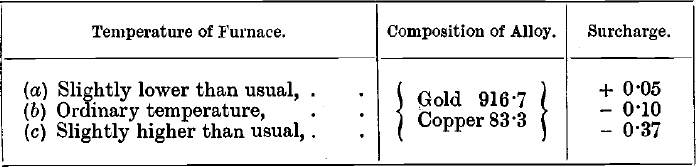
Effect of Varying Temperature
Solutions similar to the last were titrated and gave the following results:—
Effect of Varying Bulk
As before, 20 c.c. of the iron solution, and 10 c.c. of the dilute acid were diluted to the required volumes and titrated.
![]()
The variation due to difference in bulk here, although only equal to an excess of 0.7 milligram of iron for each 100 c.c. of dilution, are about three times as great as those observed in a sulphuric acid solution.
Effect of Free Hydrochloric Acid
In these experiments 20 c.c. of the ferrous chloride solution were used with varying quantities of acid, the bulk of the assay in each case being 100 c.c.
![]()
The last had a very indistinct finishing point, the brown coloration being very evanescent. The effect of the acid is modified by the presence of alkaline and other sulphates, but not by sulphuric acid. Repeating the last experiment we got—

The results with these salts, in counteracting the interference of the acid, however, were not a complete success, since the end- reactions were all indistinct, with the exception, perhaps, of that with the manganese sulphate.
Effect of Varying Amounts of Iron
In these experiments the bulk of the assay was 100 c.c., and 10 c.c. of acid were present.

In making himself familiar with the permanganate of potassium titration, the student should practise by working out a series of experiments similar to the above, varying his conditions one at a time so as to be certain of the cause of any variation in his results. He may then proceed to experiment on the various methods of reduction.
A solution of ferric chloride is made by dissolving 5.01 grams of iron wire in 50 c.c. of hydrochloric acid (sp. g. 1.16), and running from a burette nitric acid diluted with an equal volume of water into the boiling iron solution, until the liquid changes from a black to a reddish-yellow. About 4.5 c.c. of the nitric acid will be required, and the finishing point is marked by a brisk effervescence. The solution of iron should be contained in an evaporating dish, and boiled briskly, with constant stirring. There should be no excess of nitric acid. Boil down to about half its bulk; then cool, and dilute to one litre with water. Twenty c.c. of this solution diluted to 100 c.c. with water, and acidified with 10 c.c. of dilute hydrochloric acid, should not decolorise any of the permanganate of potassium solution; this shows the absence of ferrous salts. And 20 c.c. of the same solution, boiled with 20 c.c. of the ferrous sulphate solution, should not decrease the quantity of “ permanganate” required for the titration of the ferrous sulphate added. In a series of experiments on the various methods of reduction, the following results were got. The modes of working were those already described.
- With Stannous Chloride.—Twenty c.c. of the ferric chloride solution required, after reduction with stannous chloride, 20 c.c. of “ permanganate.” Fifty c.c. of a solution of ferrous chloride, which required on titration 49.8 c.c. of “permanganate,” required for re-titration (after subsequent reduction with stannous chloride) 50 c.c. of the permanganate solution.
- With Sulphuretted Hydrogen.—Two experiments with this gas, using in each 20 c.c. of the ferric chloride solution, and 10 c.c. of hydrochloric acid, required (after reduction) 20.2 c.c. and 20.1 c.c. of “ permanganate.” Repeating the experiments by passing the gas through a nearly boiling solution, but in other respects working in the same way, 21.3 c.c. and 21.6 c.c. of the permanganate solution were required. The sulphur was not filtered off in any of these. In another experiment, in which 50 c.c. of the ferrous sulphate solution were titrated with “permanganate,” 48 c.c. of the latter were required. The titrated solution was next reduced with sulphuretted hydrogen, brought to the same bulk as before, and again titrated; 47.9 c.c. of the permanganate of potassium solution were required.
- With Sodium Sulphite.—Twenty c.c. of the ferric chloride solution, reduced with sodium sulphite, required 19.9 c.c. of “ permanganate.” In one experiment 50 c.c. of the ferrous sulphate solution were titrated with “ permanganate ”; 49.3 c.c. of the last mentioned solution were required. The titrated solution was reduced with sodium sulphite, and again titrated; it required 49.2 c.c. of the permanganate of potassium solution.
- With Zinc.—Twenty c.c. of the ferric chloride solution, reduced with zinc and titrated, required 20.8 c.c. of “ permanganate.” Fifty c.c. of a solution of ferrous sulphate which required 49.7 c.c. of “ permanganate,” required for re-titration, after reduction with zinc, 49.7 c.c.
The student should next practise the titration with bichromate, which is more especially valuable in the estimation of hydrochloric acid solutions. The following experiments are on the same plan as those already given. In each experiment (except when otherwise stated) there were present 20 c.c. of the ferrous chloride solution, and 10 c.c. of dilute hydrochloric acid, and the bulk was 300 c.c.
Effect of Varying Temperature.—The quantities of the bichromate of potassium solution required were as follows :—
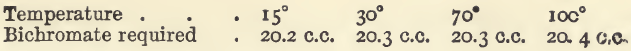
Effect of Varying Bulk:—
![]()
Effect of Varying Acid.—In these, variable quantities of dilute hydrochloric acid were used.
![]()
Effect of Foreign Salts.—The effect of the addition of 10 grams of crystallized zinc sulphate was to decrease the quantity of “bichromate” required from 20.3 c.c. to 20.1 c.c., but the colour produced with the test-drop was very slight at 18.5 c.c., and with incautious work the finishing point might have been taken anywhere between these extremes. Zinc should not be used as a reducing agent preliminary to a “bichromate” titration. Ten grams of ammonic sulphate had the effect of rendering the finishing point faint for about 0.5 c.c. before the titration was finished, but there was no doubt about the finishing point when allowed to stand for a minute. The student should note that a titration is not completed if a colour is developed on standing for five or ten minutes. Ten grams of sodic sulphate had no effect; 20.3 c.c. were required.
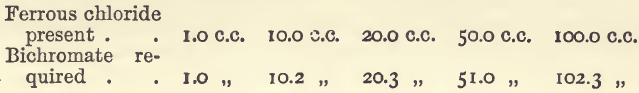
Effect of Varying Iron.—The results are proportional, as will be seen from the following details:—
The student may now apply these titrations to actual assays of minerals. The following examples will illustrate the mode of working and of calculating the results:—
Determination of Iron in Chalybite
Weigh up 1 gram of the dry powdered ore, and dissolve in 10 c.c. of dilute sulphuric acid and an equal volume of water with the aid of heat. Avoid evaporating to dryness. Dilute and titrate. The result will give the percentage of iron existing in the ore in the ferrous state. Some ferric iron may be present. If it is wished to determine this also add (in dissolving another portion) 10 c.c. of dilute hydrochloric acid to the sulphuric acid already ordered, and reduce the resulting solution before titrating. By dissolving and titrating (without previous reduction) one has a measure of the ferrous iron present; by dissolving, reducing, and then titrating, one can measure the total iron; and as the iron exists in only two conditions, the total iron; less the ferrous iron, is the measure of the ferric iron.
Determination of Iron in Brown or Red Ores or Magnetite
Weigh up 0.5 gram of the ore (powdered and dried at 100° C.), and dissolve in from 10 to 20 c.c. of strong hydrochloric acid, boiling until all is dissolved, or until no coloured particles are left. Dilute, reduce, and titrate.
Determination of Iron in Pyrites
Weigh up 1 gram of the dry powdered ore, and place in a beaker. Cover with 10 c.c. of strong sulphuric acid, mix well by shaking, and place on the hot plate without further handling for an hour or so until the action has ceased. Allow to cool, and dilute to 100 c.c. Warm until solution is complete. Reduce and titrate.
Determination of Iron in Substances Insoluble in Acids
Weigh up 1 gram of the ore, mix with 5 or 6 grams of carbonate of soda and 0.5 gram of nitre by rubbing in a small mortar, and transfer to a platinum crucible. Clean out the mortar by rubbing up another gram or so of soda, and add this to the contents of the crucible as a cover. Fuse till tranquil. Cool. Extract with water. If the ore carries much silica, evaporate to dryness with hydrochloric acid to separate it. Re-dissolve in hydrochloric acid, and separate the iron by precipitating with ammonia and filtering. If only a small quantity of silica is present, the aqueous extract of the “ melt” must be filtered, and the insoluble residue washed and dissolved in dilute hydrochloric acid. Reduce and titrate.
A convenient method of at once separating iron from a solution and reducing it, is to add ammonia, pass sulphuretted hydrogen through it, filter, and dissolve the precipitate in dilute sulphuric acid. The solution, when boiled free from sulphuretted hydrogen, is ready for titrating.
STANNOUS CHLORIDE PROCESS
The colour imparted to hot hydrochloric acid solutions by a trace of a ferric compound is so strong, and the reducing action of stannous chloride is so rapid, that a method of titration is based upon the quantity of a standard solution of stannous chloride required to completely decolorise a solution containing ferric iron. This method is more especially adapted for the assay of liquors containing much ferric iron and of those oxidised ores which are completely soluble in hydrochloric acid. It must be remembered, however, that it only measures the ferric iron present, and when (as is generally the case) the total iron is wanted, it is well to calcine the weighed portion of ore previous to solution in order to get the whole of the iron into the higher state of oxidation, since many ores which are generally supposed to contain only ferric iron carry a considerable percentage of ferrous.
The stannous chloride solution is made by dissolving 20 grams of the commercial salt (SnCl2.2H2O) in 100 c.c. of water with the help of 20 c.c. of dilute hydrochloric acid, and diluting to a litre. The solution may be slightly opalescent, but should show no signs of a precipitate. The strength of this is about equivalent to 1 gram of iron for each 100 c.c. of the solution, but it is apt to lessen on standing, taking up oxygen from the air, forming stannic chloride. A larger proportion of hydrochloric acid than is ordered above would remove the opalescence, but at the same time increase this tendency to atmospheric oxidation, as the following experiments show. The stannous chloride solution (20 c.c.) was mixed with varying amounts of strong hydrochloric acid (sp. g. 1.16), diluted to 100 c.c., and exposed in open beakers for varying lengths of time; and the residual stannous chloride measured by titration with permanganate. The quantities required were as follows:—
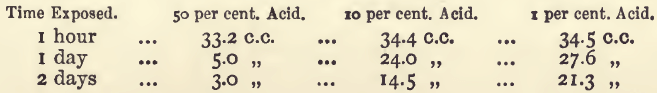
These indicate very clearly the increased susceptibility to oxidation in strongly acid solutions.
A standard solution of ferric chloride is prepared in the same manner as that described under the experiments on the methods of reduction; but it should be of twice the strength, so that 100 c.c. may contain 1 gram of iron. This solution is used for standardising the stannous chloride when required; and must be carefully prepared; and tested for the presence of nitric acid.
The titration is more limited in its application than either of the oxidising processes because of the restrictions as to bulk, quality and quantity of free acid present, and other conditions of the solution to be assayed. The following experiments show the conditions necessary for a successful titration.
Effect of Varying Temperature
Twenty c.c. of ferric chloride solution with 20 c.c. of strong hydrochloric acid, diluted to 50 c.c., gave the following results when titrated :—
![]()
The finishing point, however, is more distinct the hotter the solution; so that it is best in all cases to run the standard into the boiling solution.
Effect of Varying Bulk
Solutions containing the same quantity of iron and acid as the last, but diluted to various bulks, and titrated while boiling, gave the following results:—
![]()
Effect of Varying Quantities of Hydrochloric Acid
In these experiments the bulk before titration was 50 c.c. except in the last, in which it was 70 c.c. With less than 5 c.c. of strong hydrochloric acid the finishing point is indistinct and prolonged.
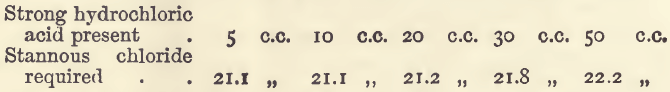
Effect of Free Sulphuric Acid
In these experiments 20 c.c, of hydrochloric acid were present, and the bulk was 50 c.c.

This interference of strong sulphuric acid may be completely counteracted by somewhat modifying the mode of working. Another experiment, like the last of this series, required 21.6 c.c.
Effect of Foreign Salts
Experiments in which 10 grams of various salts were added showed them to be without effect. The results were as follows :—

Effect of Varying Iron
Titrating a solution (with 20 c.c. of hydrochloric acid) measuring 50 c.c., and kept boiling, the quantity of stannous chloride solution required is practically proportional to the iron present.

The student, having practised some of the above experiments, may proceed to the assay of an iron ore.
Determination of Iron in Brown Iron Ore
Weigh up 1 gram of the dried and powdered ore, calcine in the cover of a platinum crucible, and dissolve up in an evaporating dish with 20 c.c. of strong hydrochloric acid. When solution is complete, dilute to 50 c.c. after replacing any acid that may have been evaporated. Boil, and run in the stannous chloride solution until the colour is faintly yellow; boil again, and continue the addition of the stannous chloride solution, stirring continuously until the solution appears colourless. Note the quantity of the stannous chloride solution required. Suppose this to be 59 c.c. Take 60 c.c. of the standard ferric chloride solution, add 20 c.c. of hydrochloric acid, boil and titrate in the same way as before. Suppose this to require 61 c.c. Then as 61 is equivalent to 60 of the iron solution, 59 is equivalent to 58.13. This gives the percentage. It is not necessary to standardise the stannous chloride solution in this way with each sample assayed, the ratio 61 : 60 would serve for a whole batch of samples; but the standardising should be repeated at least once each day.
COLORIMETRIC METHOD
This method is valuable for the determination of small quantities of iron present as impurities in other metals or ores. It is based on the red coloration developed by the action of potassic sulphocyanate on acid solutions of ferric salts.
Standard Ferric Chloride Solution.—Take 1 c.c. of the ferric chloride solution used for standardising the stannous chloride solution, add 2 c.c. of dilute hydrochloric acid, and dilute to 1 litre with water. 1 c.c. = 0.01 milligram.
Solution of Potassic Sulphocyanate.—Dissolve 60 grams of the salt in water, and dilute to a litre. It should be colourless: Use 10 c.c. for each test.
The quantity of the substance to be weighed for the assay should not contain more than a milligram of iron; consequently, if the ore contain more than 0.1 per cent, of that metal, less than a gram of it must be taken.
The method is as follows:—Weigh up 1 gram of the substance and dissolve in a suitable acid; dilute; and add permanganate of potash solution until tinted. Boil for some time and dilute to 100 c.c. Take a couple of Nessler tubes, holding over 100 c.c., but marked at 50 c.c.; label them “ 1 ” and “ 2 and into each put 10 c.c. of the potassic sulphocyanate solution and 2 c.c. of dilute hydrochloric acid. The solutions should be colourless. To “ 1 ” add 10 c.c. of the assay solution, and dilute to the 50 c.c. mark. To the other add water, but only to within 5 or 10 c.c. of this mark. Now run in the standard ferric chloride solution from a small burette, 1 c.c. at a time, stirring after each addition till the colour is nearly equal to that of the assay (No. 1). At this stage bring the solution to the same level by diluting, and make a further addition of the standard ferric chloride solution till the colours correspond. The amount of iron will be the same in each tube; that in the standard may be known by reading off the volume from the burette and multiplying by 0.01 milligram.
If the 10 c.c. of the assay solution gave a colour requiring more than 5 or 6 c.c. of the standard ferric chloride solution, repeat the determination, taking a smaller proportion.
The effect of varying conditions on the assay will be seen from the following experiments :—
Effect of Varying Temperature.—The effect of increase of temperature is to lessen the colour; in fact, by boiling, the colour can be entirely removed. All assays are best carried out in the cold.
1 c.c. at 15° would only show the colour of 0.75 c.c. at 45°
2 c.c. at 15° would only show the colour of 1.75 c.c. at 45°
5 c.c. at 15° would only show the colour of 4.0 c.c. at 45°
Effect of Time.—The effect of increase of time is to increase the colour, as will be seen from the following experiments:—
2 c.c. on standing 10 minutes became equal to 2.25 c.c.
2 c.c. on standing 20 minutes became equal to 2.75 c.c.
2 c.c. on standing 40 minutes became equal to 3.00 c.c.
Effect of Free Acid.—If no acid at all be present, the sulpho-cyanate of potassium solution removes the colour it first produces, so that a certain amount of acid is necessary to develop the colour. The use of a large excess has a tendency to increase the colour produced.
5 c.c. nitric acid (sp. g. 1.4) read 3.7 c.c. instead of 2 c.c. with the dilute acid.
5 c.c. sulphuric acid (sp. g. 1.32) read 2.2 c.c. instead of 2 c.c. with the dilute acid.
5 c.c. hydrochloric acid (sp. g. 1.16) read 2.5 c.c. instead of 2 c.c. with the dilute acid.
Effect of Foreign Metals.—Lead, mercury, cadmium, bismuth, arsenic, tin, antimony, nickel, cobalt, manganese, aluminium, zinc, strontium, barium, calcium, magnesium, sodium, or potassium, when separately present in quantities of from 100 to 200 times the weight of iron present, do not interfere if they have previously been brought to their highest oxidised condition by boiling with nitric acid or by treating with permanganate. Arsenic and phosphoric acids interfere unless an excess of free hydrochloric or other acid is present. Oxalic acid (but not tartaric acid) in minute quantities destroys the colour. Nitrous acid strikes a red colour with the sulphocyanate of potassium; consequently, when nitric acid has been used in excess, high results may be obtained. Copper and some other metals interfere, so that in most cases it is advisable to concentrate the iron before estimating it. A blank experiment should always be made with the reagents used in order to determine the iron, if any, introduced during the solution, &c., of the substance assayed.
Determination of Iron in Metallic Copper.—This may be most conveniently done during the estimation of the arsenic. The small quantity of white flocculent precipitate which may be observed in the acetic acid solution before titrating, contains the whole of the iron as ferric arsenate. It should be filtered off, dissolved in 10 c.c. of dilute hydrochloric acid, and diluted to 100 c.c.; 10 c.c. of this may be taken for the estimation. For example: 10 grams of copper were taken, and the iron estimated; 3. c.c. of standard ferric chloride solution were used, equivalent to 0.03 milligram of iron; this multiplied by 10 (because only 1/10-th of the sample was taken) gives 0.3 milligram as the iron in 10 grams of copper. This equals 0.003 per cent.
In a series of experiments with this method working on 10-gram lots of copper, to which known quantities of iron had been added, the following were the results:—
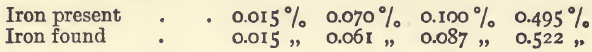
When no arsenic is present in the copper, the iron can be separated by fractionally precipitating with sodic carbonate, dissolving in ammonia, and filtering off the ferric hydrate. Coppers generally carry more iron the less arsenic they contain.
Determination of Iron in Metallic Zinc
Dissolve 1 gram of zinc in 10 c.c. of dilute hydrochloric acid, adding a drop or two of nitric acid towards the end to effect complete solution. Boil, dilute, and tint with the permanganate of potassium solution; boil till colourless, and dilute to 100 c.c. Take 10 c.c. for the determination. Make a blank experiment by boiling 10 c.c. of dilute hydrochloric acid with a drop or two of nitric acid; add a similar quantity of the permanganate of potassium solution, boiling, &c., as before. The quantity of iron in zinc varies from less than 0.005 to more than 2.0 per cent. When 1 gram is taken and worked as above, each c.c. of ferric chloride solution required indicates 0.01 per cent., of iron.
Determination of Iron in Metallic Tin
Cover 1 gram of tin with 5 c.c. of hydrochloric acid, add 1 c.c. of nitric acid, and evaporate to dryness. Take up with 2 c.c. of dilute hydrochloric acid, add 10 c.c. of the potassic sulphocyanate solution, and make up to 50 c.c. Probably the colour developed will be brown instead of red owing to the presence of copper: in this case, add to the standard as much copper as the assay is known to contain (which must have previously been determined; see Copper); the titration is then carried out in the usual way.
Or the iron may be separated from the copper in the tin by the following process:—Dissolve 5 grams of metal in 30 c.c. of hydrochloric acid and 5 c.c. of nitric acid, and evaporate to dryness. Take up with 5 c.c. of dilute hydrochloric acid, add 10 grams of potash dissolved in 30 c.c. of water, and warm till the tin is dissolved. Pass sulphuretted hydrogen, boil, cool, and filter. The iron and coppor will be in the precipitate. They are separated in the ordinary manner.
PRACTICAL EXERCISES
1. Calculate from the following determinations the percentages of ferrous, ferric, and total iron in the sample of ore used.
1 gram of ore dissolved and titrated required 26.7 c.c. of bichromate of potassium solution.
1 gram of ore dissolved, reduced, and titrated required 43.5 c.c. of bichromate of potassium solution.
Standard = 1.014.
2. One gram of an ore contained 0.307 gram of ferrous iron and 0.655 gram of total iron. The iron existing as oxide, what are the percentages of ferrous oxide (FeO) and ferric oxide (Fe2O3) in the ore ?
3. One gram of brown iron ore dissolved in hydrochloric acid required 59.2 c.c. of stannous chloride (standard=0.930). Another gram dissolved in acid and titrated with “ permanganate ” required
8.2 c.c. (standard=o.4951). Calculate the percentages of ferrous, ferric, and total iron.
4. Another gram of the same ore, roasted, dissolved and titrated with stannous chloride, required 63.5 c.c. To what extent does this result confirm the others ?
5. Two grams of a metal were dissolved and diluted to 100 c.c. Five c.c. were taken for a colorimetric determination, and required 4.5 c.c. of the standard ferric chloride solution. What is the percentage of iron in the metal ?
