Tin occurs in nature as cassiterite (containing from 90 to 95 per cent, of oxide of tin), which mineral is the source from which the whole of the tin of commerce is derived. Tin also occurs as sulphide combined with sulphides of copper and iron in the mineral stannine or bell-metal ore. It is a constituent of certain rare minerals, such as tantalite.
The methods of assaying tin in actual use are remarkable when compared with those of other metals. The more strictly chemical methods are rendered troublesome by the oxide being insoluble in acids, resembling in this respect the gangue with which it is associated. Moreover, it is not readily decomposed by fusion with alkalies. The oxide has first to be reduced to metal before the tin can be dissolved. The reduction may be performed by fusing with potassic cyanide, by heating to moderate redness in a current of hydrogen or coal gas, or by heating to a higher temperature with carbon. The reduced metal is only slowly dissolved by hydrochloric acid, and although it is readily soluble in aqua regia, the solution cannot be evaporated or freed from the excess of acids, by boiling, without loss of tin, because of the volatility of stannic chloride. There has long been a difficulty in getting a quick wet method.
The process of assaying tin ores adopted in the mines of Cornwall is a mechanical one known as “ vanning,” the object of which is to find the percentage of “ black tin,” which, it is well to remember, is not pure cassiterite, much less pure oxide of tin. Tin ore, as taken from the lode, contains from 2 to 5 per cent, of cassiterite, and is mainly made up of quartz, felspar, chlorite, schorl, and other stony minerals, together with more or less mispickel, iron and copper pyrites, oxide of iron, and wolfram. The cassiterite has a specific gravity (6.4 to 7.1) considerably higher than that of the vein-stuff (2.5 to 3.0), and is concentrated by a series of washings till it is free from the lighter material. Those minerals which have a specific gravity approaching that of the cassiterite are not completely removed. The mispickel and copper and iron pyrites are converted into oxides by roasting, and are in great part removed by a subsequent washing. The concentrated product is known as “black tin,” and in this condition is sold to the smelter. The chief foreign matters in the black tin are silica, oxides of iron and copper, and wolfram, with traces of manganese and niobic acid ; and in certain stream ores there may be as much as 6 or 7 per cent, of titaniferous iron. The black tin from the mines contains from 5 to 12 per cent, of water, and sold and assayed wet. A series of typical samples of black tin ranged as follows :—
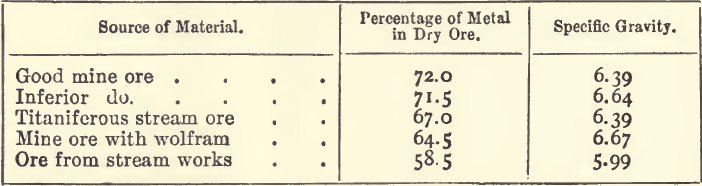
It will be seen from these figures that black tin is a very variable substance; and that the specific gravity is largely influenced by the impurities; hence, it is only an indication of the percentage of metal when the same kind of ore is dealt with.
As already pointed out, the object of vanning is to determine the proportion of black tin in the lode stuff. The relation between the actual content in oxide of tin and the produce got by vanning has been tested on several occasions with results which show a fair degree of approximation.
The following are some published results of assays of the same batch of ore. The vanning results were obtained by a Cornish vanner of recognised ability, and the wet assays by two London firms of the highest standing :—

The vanner reported his black tin as containing 70 per cent, of tin. This will bring his result, if calculated as stannic oxide, to 80.9 lbs. to the ton ; which agrees with the others.
According to our experience the “ van ” assay agrees fairly well with the “ wet ” one, if the black tin is assumed to contain 92.5 per cent, of stannic oxide (SnO2).
Vanners are, as a rule, skilful men, and show remarkable dexterity in separating the black tin, with the help of their apparatus, which consists simply of a shovel and a kieve of water. An account of the process is given below. But different vanners, all good men, will get different results working on material new to them. The black tin weighed by the vanner is supposed to correspond in quality with the black tin returned from the floors of the mine for which he is assaying, but this differs materially in different mines with the nature of the gangue. The process leaves too much to the judgment of the vanner. It is more than probable that in practice the returns from the dressing-floors check the assayer, instead of, as should properly be the case, the assayer checking the returns. It is only when this last is done that any control is had over the system of dressing. A correct assay of this ore is a matter of some importance, because of the high price of the metal.
The method of assaying the black tin is a dry one, and consists of mixing it with ” culm,” and submitting it in a black-lead crucible to the highest temperature of a wind furnace. The sample is taken wet as it arrives at the smelting house, and is assayed direct. The product of the assay is examined, and a deduction of a considerable percentage is very properly made for impurities, since the assay really determines the percentage, not merely of tin, but of the bodies present which are reducible at a white heat. The judgment as to how much is to be deducted is assisted partly by an examination of the metal got from the assay, and partly by the experience acquired in smelting similar ores. The produce, which is that of the impure tin, is stated in parts in twenty; thus a produce of 14 is equivalent to 70 per cent., or to 14 cwt. per ton.
VANNING
This process, which has already been referred to, is carried out as follows:—After sampling the ore in the ordinary way, a quantity (varying with its richness) is weighed out. Special weights are generally used. The standard weight, marked 200, weighs about an ounce; with poor ores this quantity is taken for an assay, but with richer ores 100 or even 50 is sufficient. The unit of weight has no special name, but the parts in 200 are spoken of as the produce; thus, if 200 of ore were taken and 9.5 of black tin were separated, the produce would be 9½ : obviously half the “ produce ” will give the percentage. The weighed portion of the ore is placed on the vanning shovel. The vanner stands in front of a tub of water (kieve) and allows 30 or 40 c.c. of water to flow on to the ore. He then raises the shovel a little above the surface of the water, and, holding it nearly horizontal, briskly rotates the water by imparting to the shovel a slight circular motion, passing into an elliptical one (front to back). This causes the finer mud to be suspended in the liquid, which is then run off, leaving the body of the ore in the centre of the shovel. This is repeated until the water after standing a moment is fairly clear. About half as much water as before is brought on; then, with a motion which is similar to the previous one, but with a jerk added in one direction, the heavier minerals are thrown up, and the stony matter brought back. The jerk is produced just as the wave of water is returning. The descending wave of water draws with it the bulkier and lighter particles of the ore, whilst the heavier matter lying on the bottom is scarcely affected by it. The jerky motion, however, carries it to the front of the shovel. The lighter stuff is washed off, and the residue dried by holding the shovel over the furnace. It now corresponds, more or less, to the stuff which on the mine is sent to the calciner. It is swept from the shovel into a scoop, and transferred to a hot  crucible; in which it is calcined until free from sulphur. Some vanners calcine their samples before commencing to van. The calcined ore is shaken out of the crucible on to the shovel; rubbed up with a hammer; and washed (as at first) to get rid of the finer and lighter “ waste.” The separating motions are again gone through; and the “ head ” of the best of the black tin is thrown well up on one side of the shovel in the form of a crescent, so as to leave room on the shovel to work with the “ tailings.” The quantity of water used is kept low, to prevent this “ crop ” tin from being washed back again. The tailings are then crushed to free the tin from adherent oxide of iron; and again washed to throw up the remaining tin ore. As this tin is finely divided, it is more difficult to bring it up, so that a vigorous and rapid motion is required. The tailings are now washed off, and the whole of the black tin is brought into the centre of the shovel. It requires two or three washings more to free it from the waste it contains. Very small quantities of water are used. The purity of the black tin can be seen by its appearance on the shovel. The cleaned ore is dried as before, freed from particles of iron with the aid of a magnet, and weighed. The weighings are carried to 1/8th of the unit used. The following example illustrates the method of calculation adopted on the mine. A parcel of 1 ton 2 cwt. 3 qrs. of tin ore with a produce of 45 (equal to 22½ per cent.) contains 5 cwt. o qrs. 12 lbs. of black tin. This result is obtained as follows:—
crucible; in which it is calcined until free from sulphur. Some vanners calcine their samples before commencing to van. The calcined ore is shaken out of the crucible on to the shovel; rubbed up with a hammer; and washed (as at first) to get rid of the finer and lighter “ waste.” The separating motions are again gone through; and the “ head ” of the best of the black tin is thrown well up on one side of the shovel in the form of a crescent, so as to leave room on the shovel to work with the “ tailings.” The quantity of water used is kept low, to prevent this “ crop ” tin from being washed back again. The tailings are then crushed to free the tin from adherent oxide of iron; and again washed to throw up the remaining tin ore. As this tin is finely divided, it is more difficult to bring it up, so that a vigorous and rapid motion is required. The tailings are now washed off, and the whole of the black tin is brought into the centre of the shovel. It requires two or three washings more to free it from the waste it contains. Very small quantities of water are used. The purity of the black tin can be seen by its appearance on the shovel. The cleaned ore is dried as before, freed from particles of iron with the aid of a magnet, and weighed. The weighings are carried to 1/8th of the unit used. The following example illustrates the method of calculation adopted on the mine. A parcel of 1 ton 2 cwt. 3 qrs. of tin ore with a produce of 45 (equal to 22½ per cent.) contains 5 cwt. o qrs. 12 lbs. of black tin. This result is obtained as follows:—

Similarly, a parcel of 20 tons 10 cwt. with a produce of 9½ contains 19 cwt. 1 qr. 25 lbs. of black tin. For the following information, as well as for much of that already given about vanning, we are indebted to Captain Reynolds, of Cook’s Kitchen Mine. “ To have a complete set of tools for all vanning purposes, it will be necessary to get the following:—A vanning shovel 14 inches long and 13 inches wide, weighing not over 2¾ pounds. It is made of hammered sheet iron of the shape shown in fig, 57. It must have a light wooden handle (preferably of deal) 3 feet long. A bruising hammer, weighing 2½ pounds, with a handle 1 foot long. A pair of tongs (furnace) 2½ feet long, made of ½-inch round iron. And a set of ordinary clay crucibles for calcining. There ought to be two sets of scales and weights: the first should be confined to weighing the powdered tin stuff, and the second ought to be a much higher class one, for weighing the black tin obtained. The furnace for roasting the sample should be 10 inches square and 12 inches deep, with the fire-bars at the bottom three- quarters of an inch apart. The water-box for vanning in should be at least 4 feet long, 2 feet 6 inches wide, and 8 inches deep.”
DRY METHODS
For the following description of the process adopted in Cornwall we are indebted to Mr. A. K. Barnett, F.G.S., of Chyandour.
Cornish Method
Tin Ore Assay.—The ore to be smelted or assayed should be concentrated to say not less than 50 per cent, of metallic tin; though to obtain satisfactory results it should be brought nearer 70 per cent., as with ore containing less than 40 to 50 per cent, of metal there will be a considerable loss both in the assaying and in the smelting. If the ore to be operated on does not contain this quantity of metal, then the sample (if coarse) must be reduced to a fine state, the gangue being removed by vanning, and the ore saved for the fire assay.
The method adopted for the determination of tin in the ore is as follows:—About 2½ ounces troy (1200 grains, or about 80 grams) of the ore to be assayed is weighed out and mixed on a flat copper pan (shaped with a long lip) with one-fifth of its weight (240 grains, or 15.5 grams) of powdered culm (anthracite). The mixture of ore and culm is either transferred to a black-lead crucible before the latter is put into the furnace, or, as some prefer, it is carefully swept into a crucible which has been imbedded in the fire. Some assayers cover their pots with a flat cover placed loosely on, while others leave the mixture in the open pot. The furnace, which has been previously fired to a strong heat, is then covered, and the sample is subjected to a sharp fire for a period of from twelve to twenty minutes. No definite time can be stated, as, besides the strength of the fire, the quality and condition of the ore, and the impurities associated with it, greatly affects the time required for the complete reduction of the ore. As soon as the mixture in the crucible has settled down to a uniform white heat, and any very slight ebullition which may have taken place has subsided, the crucible is gently shaken, removed from the fire (the culm-ash or slag which covers the metal being carefully drawn aside with an iron scraper), and the metal is poured quickly into an iron ingot-mould, which is usually placed on a copper pan to save the culm-slag and the adherent metal which comes out with it. The crucible is then carefully scraped, and the scrapings, together with the contents of the mould and pan, are transferred to a mortar. There the ingot of tin is freed from slag and then taken to the scales. The rest, after being finely powdered, is passed through a sieve. The flattened particles of tin which remain on the sieve are weighed with the ingot (the lump, as it is called) 3 whilst the siftings are vanned on a shovel, and (the slag being washed off) the fine tin is collected, dried, and weighed with the rest: the whole gives the produce or percentage of metal in the ore. The results of the assays are expressed in cwts. of metal in the ton of ore. The percentage is rarely given and never used in Cornwall. Thus— “ 13½ Produce ” would mean that the assay yielded results at the rate of 13½ cwts. of metal for one ton of the ore. Some assayers use a little powdered fluor-spar to assist the fusion of refractory slags. A small quantity of borax will also occasionally be of service for ores containing silica in excess of any iron that may be present. The borax renders the slag more fusible, and assists the formation of a larger lump (with less fine tin in the slag) than would be obtained by the use of culm alone.
The quality and the percentage of pure tin in the metal will vary considerably, according to the impurities that are associated with the ore to be assayed.
The crude lump is then remelted in a small iron ladle at as low a temperature as possible, and the fused metal is poured into a shallow trench about 4 inches long by ¾ of an inch wide cut in a block of white marble. The metal will be silvery-white if the temperature employed be correct; if too hot, the surface will show a yellow, red, or blue colour (according to the heat employed) ; in such case the metal should be remelted at a lower temperature. If the metal on cooling remains perfectly clear and bright, then it may be assumed that the tin is of good quality and commercially pure. A crystallised or frosted appearance of the metal indicates the presence of some alloy, say of iron, copper, zinc, lead, antimony, &c. The assayer who has had much practice can readily distinguish the metal or metals that are associated with the ore by noting the appearance of the tin on cooling; and can fairly judge the quantity of impurity present by the amount of the crystallisation or stain.
Whilst the foregoing method of assaying cannot lay claim to scientific accuracy, it is by no means so imperfect as some writers would have us believe, who state that a loss of 5 to 10 per cent, arises in the operation. It is certainly the most ready and expeditious mode of determining the commercial value of a parcel of tin ore, which, after all, is the main object of all assaying operations.
The difficulty which beginners find in obtaining satisfactory results, and any loss of metal which those not accustomed to the process may incur, will invariably occur in the vanning of the powdered slag for the fine tin, the rest of the operations being easy of execution, and requiring only the ordinary care necessary for all metallurgical work.
There is no doubt that if low percentage ores containing silica are assayed in this manner, low results are obtained, as it is impossible to reduce the whole of the tin in the presence of free silica; with this class of ores, care should be taken to remove some of the silica by preliminary vanning, or some flux should be added which will combine with the silica, and so prevent its entering into combination with the tin. Low quality tin ores containing iron, copper, lead, zinc, antimony, &c., combined with arsenic, sulphur, or oxygen, will give very much higher results than the actual percentage of tin in the sample. The other metals (being readily reduced in the presence of tin) alloy with it, and give a hard lump difficult to fuse in the iron ladle; where the quantity of foreign metals is large, the metal can only be melted to a stiff pasty mass; so that (in determining the value of a ton of tin ore, or even reporting on the percentage of tin it contains) not only must the weight of the assay be the basis for calculation, but the quality and character of the metal obtained must also be considered. Thus two ores of tin might be assayed both yielding a similar produce, say 13½ (67½ per cent.), and yet one might contain 5 per cent, less tin than the other.
If it be required to obtain the pure metal from tin ores containing the ores of other metals associated with them, the latter must be removed by digesting in strong hydrochloric acid, and washing. The assay may then be conducted in the usual way, and a fairly pure lump will be obtained.
If wolfram be present in any appreciable quantity in the ore, it considerably reduces the proportion of lump, and at the same time it increases the fine tin (or prillion, as it is termed) in the assay. This may be got rid of by boiling in aqua regia, and dissolving out the tungstic acid which has been liberated by means of ammonia.
It will be seen that this method of assaying tin has its advantages and its drawbacks. It is quickly performed; with ores of good quality it gives results not to be excelled by any other process; and it gives the smelter the actual alloy and quality of metal he may expect to get in the smelting of the ore, which no other mode of assaying will do: against which may be set the skill required to obtain accurate results with the vanning shovel; the loss of metal in poor ores containing an excess of silica; and the high results from ores containing a large quantity of metallic impurities.
Cyanide Method
Weigh up 20 grams of the ore and dry it on a scoop over the Bunsen flame. When dry, weigh, and calculate the percentage of water from the loss in weight. Transfer the dried ore to an evaporating dish, and cover with 30 c.c. of hydrochloric acid: boil for 10 or 12 minutes, and then add 5 c.c. of nitric acid and boil again. Dilute with water, and filter. Transfer the filter and its contents to an E Battersea crucible, and calcine it for a few minutes. Cool, and weigh the residue. The loss equals the oxides soluble in acid. Transfer the residue to the crucible and mix it with its own weight of cyanide of potassium; add a similar amount of “ cyanide ” as a cover. Place in the furnace, and when the charge has attained the temperature of the furnace (in from 3 to 6 minutes), remove it at once ; tap the pot vigorously several times, and then pour its contents quietly into a mould. Dissolve the slag in water, clean, dry, and weigh the button of tin.
WET METHODS
Detection
Tin ore is detected by its insolubility in acids, high specific gravity, and characteristic appearance in water. The powder is separated from the lighter gangue by washing. It is fused in a Berlin crucible with five times its weight of potassic cyanide at a moderately high temperature in a muffle, or over the blowpipe. The slag is washed off with water, and the metallic buttons or residue treated with hydrochloric acid (not aqua regia), for some time. One portion of the solution strikes a purple colour with chloride of gold, another portion gives a white or grey precipitate or cloudiness with mercuric chloride. These reactions are characteristic of tin as stannous chloride.
Metallic tin treated with nitric acid becomes converted into a white insoluble powder (metastannic acid). Aqua regia dissolves tin readily, forming stannic chloride, and in this solution the metal is detected by precipitation with sulphuretted hydrogen, which gives a yellow precipitate. Tin in solution as stannic or stannous chloride is precipitated as metal by means of zinc.
The fact that tin forms two well-defined series of compounds is taken advantage of in assaying (just as in the case of iron), by determining how much of an oxidising agent is required to convert it from the stannous into the stannic state. For example, on the addition of a solution of permanganate of potash to a solution of stannous chloride the oxidation goes on rapidly, and the finishing point is sharp and distinct; but acid solutions of stannous chloride quickly take up oxygen from that dissolved in the water used and from the air. Unfortunately, there is no obvious sign that such oxidation has taken place, except that (fatal to the assay) a smaller volume of the permanganate is required. Great care is required with such solutions, both before and during titration. The addition of an excess of ferric chloride to the stannous solution, as soon as the whole of the tin has been dissolved, will lessen this liability to oxidation.
Separation
If the tin is present in an alloy, the substance is boiled in an evaporating dish with dilute nitric acid until the whole of the material is attacked. Evaporate nearly to dryness, dilute, boil for a few minutes, and filter off the white insoluble residue. Under certain circumstances this residue will be nearly free from other metals, in which case it is ignited and weighed. If not known to be pure it must be ignited, reduced in a current of hydrogen, and treated as subsequently described.
When the tin is present as insoluble oxide in an ore, the substance is finely powdered, and from 1 to 5 grams of it (according to its richness) boiled with 30 c.c. of hydrochloric acid in an evaporating dish till the oxide of iron is seen to be dissolved. Then add 1 c.c. of nitric acid (or more if much pyrites, &c., is present) and continue the boiling till these are decomposed; dilute and filter off, washing first with dilute acid and afterwards with a little dilute ammonia, dry, ignite, and place in a combustion tube (together with the filter-ash) and heat to redness for about thirty minutes in a current of dried hydrogen.
The oxide of tin is placed in a porcelain boat (fig. 58), which is then introduced into a piece of combustion tube. The latter,

wrapped in a piece of wire gauze, is supported on a couple of iron rings, and heated by one or two Bunsen burners in a furnace fitted up with loose fire-brick tiles, as shown in fig. 59.
When the reduction is complete the tube is allowed to cool; the boat is removed and the tin dissolved. Add a rod of zinc to the freely-acid hot solution, and in a few minutes decant through a filter and wash with water, after having removed the zinc. Wash the precipitated metal back into the beaker, and dissolve in 10 c.c. of dilute nitric acid, evaporate off the excess of acid; dilute, boil, and filter. Wash, dry, ignite strongly in a porcelain crucible, and weigh.
In the absence of antimony the above separation works very well, but if this metal is present in quantity the metals precipitated on the zinc must be covered with hydrochloric acid and treated with a few drops of nitric. It is then warmed with iron wire until no more of the latter dissolves. The antimony is precipitated as metal, and the tin remains in solution as stannous chloride. The antimony is filtered off, and may be washed with alcohol, and weighed, whilst the tin in the filtrate is precipitated with zinc, and treated as already described.
GRAVIMETRIC METHOD
If the tin is not already in the metallic state it is reduced to this condition by the method given (precipitation by zinc). Treat the finely-divided metal (washed free from chlorides) in a four- inch evaporating dish with 10 c.c. of dilute nitric acid, cover with a clock-glass, and apply a gentle heat until the precipitate appears of a white colour and the metal is completely attacked. Evaporate nearly to dryness on a water-bath; then add 50 c.c. of water, heat to boiling, and filter. Wash with hot water, dry, transfer to a weighed porcelain crucible, add the filter-ash, ignite strongly, and weigh. The precipitate after ignition is stannic oxide (SnO2). It is a yellowish-white powder (darker whilst hot), insoluble in acids, and contains 78.67 per cent, of tin. Cold dilute nitric acid dissolves tin to a clear solution, which becomes a white enamel-like jelly on heating; this (filtered off, washed, and dried) forms an opal-like substance, which is converted on ignition into stannic oxide with evolution of nitrous fumes. Stannic oxide when ignited with chlorides is more or less completely converted into stannic chloride, which volatilises. The presence of chlorides during the evaporation with nitric acid causes a similar loss.
Determination of Tin in an Alloy
(Bronze.)—Take 2 grams, and attack with 20 c.c. of dilute nitric acid in a covered beaker with the aid of heat. Boil till the bulk is reduced by one- half, dilute with 50 c.c. of water, allow to settle for a few minutes, and filter; wash well first with water acidulated with a little nitric acid, and afterwards with water; dry, ignite, and weigh as stannic oxide.
Determination of Tin in Tin Ore
Treat 5 grams of the dried and finely-powdered ore with 30 c.c. of hydrochloric acid in a four-inch evaporating dish. After the soluble oxides have been dissolved add 1 or 2 c.c. of nitric acid, boil off nitrous fumes, dilute, and filter. Dry the filter, transfer the cleaned ore to a piece of combustion tube ten or twelve inches long and narrowed at one end. Pass a Current of hydrogen through the tube and heat to redness for 30 minutes ; cool whilst the gas is still passing. Dissolve in 20 c.c. of dilute hydrochloric acid and keep the solution tinted with permanganate of potassium. When the colour of the permanganate becomes permanent dilute to a bulk of 50 c.c. with water, filter, and wash. Heat; add a rod of zinc weighing about 3 grams; allow to stand for a few minutes ; decant through a filter; and wash, removing the remaining zinc and returning the tin to the beaker. Treat with 5 c.c. of dilute nitric acid, boil for some time, take up with water, filter, wash, dry, ignite, and weigh as stannic oxide.
VOLUMETRIC METHOD
Titration with Solution of Permanganate of Potassium
This titration maybe made either directly on the solution of stannous chloride (prepared by dissolving the precipitated metal in hydrochloric acid), or indirectly, on a solution of ferrous chloride (produced by the reducing action of the precipitated metal on ferric chloride). The standard solution of permanganate of potassium is made by dissolving 5.356 grams of the salt in water and diluting to one litre. 100 c.c. are equivalent to 1.00 gram of tin.
The precipitated tin is transferred to a flask; and dissolved in 10 c.c. hydrochloric acid, with the aid of heat and in an atmosphere of carbonic acid. The acid and metal are placed in the flask; which is then filled with the gas, and stopped with a cork provided with a rubber valve. When solution is complete the flask is again filled with carbonic acid. Fifty c.c. of water freed from air and saturated with carbonic acid are then added. This water is made by adding a gram of bicarbonate of soda and 2 c.c. of hydrochloric acid to 100 c.c. of water : the effervescence sweeps out the dissolved oxygen. The permanganate of potassium solution is then run in from a stop-cock burette in the usual way until a faint pink tinge is obtained.
The following experiments show the effect of variations in the conditions of the assay. A solution of stannous chloride equivalent in strength to the “ permanganate ” was made by dissolving 19.6 grams of the crystallised salt (SnCl2.2H2O.) in 50 c.c. of water and 10 c.c. of hydrochloric acid and diluting to 1 litre with water freed from dissolved oxygen. 100 c.c. contain 1 gram of tin. In the first experiments tap water was used and no precautions were taken for excluding air. Except when otherwise stated, 20 c.c. of the stannous chloride were used in each experiment with 10 c.c. of hydrochloric acid, and were diluted to 100 c.c. with water before titration.
Effect of Varying Hydrochloric Acid
![]()
The only effect of the increase in quantity of acid was to give the brown of perchloride of manganese instead of the pink of permanganic acid to mark the finishing point.
Effect of Varying Temperature
![]()
Rate of Atmospheric Oxidation
Solutions ready for titration were exposed to air at the ordinary temperature for varying lengths of time and then titrated.
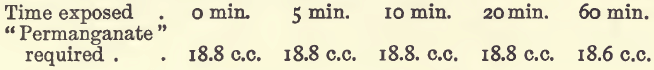
It is best to titrate at once, although the loss by oxidation is only small after one hour’s exposure.
Effect of Varying Tin
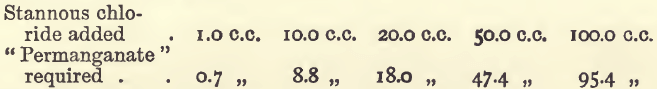
Effect of Varying Bulk
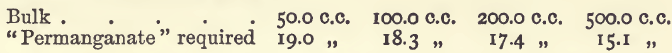
The two last series show an interference, which is due to the oxygen dissolved in the water, as may be seen from the following similar experiments, which were, however, performed with water freed from oxygen and in which the titrations were effected in an atmosphere of carbonic acid.
Effect of Varying Tin
A new solution of stannous chloride was used.

Effect of Varying Bulk
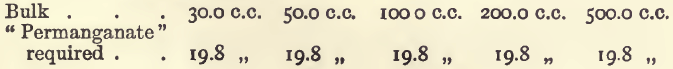
It will be seen that in working under these conditions the results are proportional and the method satisfactory.
Examination of Tin Phosphide
(Phosphor Tin.)—This substance is used in the manufacture of “ phosphor bronze ” and similar alloys. It is a crystalline, imperfectly-malleable, metallic substance. It is soluble in hydrochloric acid with effervescence ; phosphoretted hydrogen, which inflames on the addition of a drop or two of nitric acid, being evolved. It is attacked by ( nitric acid, yielding a white powder of stannic phosphate ; this is not easily decomposed by ammonium sulphide or readily soluble in hydrochloric acid.
“ Phosphor-tin ” is made up only of tin and phosphorus. For the estimation weigh up 1 gram. Place in a weighed Berlin dish; and cover with 10 c.c. of nitric acid and 3 or 4 c.c. of water. Let the reaction proceed (under a clock-glass) on the water-bath till complete. Remove the glass; evaporate to dryness, and ignite, at first gently over a Bunsen burner, and afterwards in the muffle at a red heat. Cool in the desiccator, and weigh as quickly as possible when cold. The substance contains the tin as stannic oxide, SnO2, and the phosphorus as phosphoric oxide, P2O5. The increase in weight on the gram of substance taken gives the weight of the oxygen taken up by the phosphorus and tin, and since 1 gram of tin takes up only 0.271 gram of oxygen, and 1 gram of phosphorus takes up 1.29 gram, the proportion of tin to phosphorus can be calculated from the increase in weight. For example, 1 gram of a sample gave 1.3410 gram of mixed oxides, which is 0.070 gram in excess of that which would be got with pure tin. If the substance was all phosphorus the excess would be 1.0190 gram; consequently the proportion of phosphorus in the substance is 0.070 ÷ 1.019, or 6.87 percent. The tin is calculated by difference, 93.13 per cent.
Another method of separating and determining the phosphorus is as follows :—Take 1 gram of the substance and add to it 15 c.c. of hot aqua regia. Boil till dissolved, dilute, and precipitate the tin with sulphuretted hydrogen. To the filtrate add ammonia and “ magnesia mixture.” Filter; wash the precipitate with dilute ammonia; dry, ignite, and weigh as magnesic pyrophosphate. Calculate the phosphorus, and take the tin by difference.
A sample of phosphor tin gave—
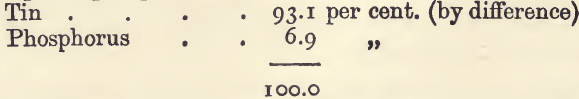
Tin Arsenide
This is met with in tin-smelting; it closely resembles the phosphide, but the crystals have a duller grey appearance. It contains simply tin and arsenic. The determination is made by treating 1 gram of the substance with nitric acid and weighing the mixed oxides of tin and arsenic in the same manner as in the case of the phosphide. One gram of arsenic will give 1.533 gram of arsenic oxide, As2O5; consequently the excess of weight of the mixed oxides over 1.271 gram must be divided by 0.262; the result multiplied by 100 gives the percentage of arsenic. In consequence of the higher atomic weight of arsenic the results by this method are not so close as with the phosphide. Each milligram of excess weight (over 1.271) represents 0.38 per cent, of arsenic, As. Both in this and in the corresponding phosphide determination care must be taken to avoid absorption of moisture, by allowing the oxides to cool in a desiccator and weighing quickly.
The percentage of arsenic is better determined as follows:— Weigh up 1 gram of the substance, dissolve in aqua regia, dilute, and pass sulphuretted hydrogen. Render alkaline with ammonia, and add ammonium sulphide till the precipitate is dissolved. Add “ magnesia mixture.’’ Filter off the precipitate, wash with dilute ammonia, ignite with a few drops of nitric acid, and weigh as magnesic pyrarsenate. Calculate the arsenic and take the tin by difference. A sample treated in this way gave—

Examination of Black Tin
Dry the ore, and reduce it to a fine powder. Weigh up 2 grams, and boil with 20 c.c. of hydrochloric acid and 2 c.c. of nitric for ten or fifteen minutes. Filter, and reserve the filtrate.
Tungstic Acid.—Digest the residue with about 50 c.c. of water and a few c.c. of dilute ammonia for a few minutes, and filter; collect the filtrate in a weighed porcelain dish, evaporate to dryness, ignite, and weigh as tungstic acid, WO3.
Stannic Oxide.—Dry, ignite, and weigh the insoluble residue. Transfer to a porcelain boat, and reduce in a current of hydrogen at a red heat for half an hour. Allow to cool whilst the hydrogen is still passing. Transfer the boat to a beaker, and dissolve up the tin in 10 c.c. of hydrochloric acid and a c.c. or so of nitric. Wash out the combustion tube with some acid and add the washing to the contents of the beaker. Warm gently, dilute with water, and filter. Collect, dry, ignite, and weigh the insoluble residue. Through the filtrate pass a rapid current of sulphuretted hydrogen, allow to settle, and filter. Wash the precipitate with hot water, dry, calcine gently; ignite with ammonium carbonate, and weigh as stannic oxide, SnO2. The insoluble residue will in most cases retain some tin. Fuse it with fusion mixture, take up with hydrochloric acid, filter, pass sulphuretted hydrogen through the filtrate, collect and wash the sulphide of tin. Ignite and weigh as stannic oxide, and add it to that previously obtained.
Copper.—Pass sulphuretted hydrogen through the acid filtrate obtained in the first cleaning of the ore, collect the precipitate, and wash first with soda solution and then with hot water. Dry, ignite, and weigh as cupric oxide, CuO. Mix the filtrate with that from the main portion of the sulphide of tin.
Ferric Oxide.—Boil off the sulphuretted hydrogen from the mixed filtrates and peroxidise with nitric acid. Add ammonia in slight excess, boil, filter, dry, ignite, and weigh the precipitate as ferric oxide. This will be practically pure, but the iron in it must be determined by dissolving and titrating. The filtrate from the iron may contain zinc, lime, and magnesia, but rarely in quantities sufficient to be determined.
Silica, &c.—The silica may be calculated from the weight of the residue insoluble in acid, after the reduction of the tin in hydrogen, by deducting from it the weight of the oxide of tin subsequently found. Or it may be determined as follows:— The insoluble portion is fused with fusion mixture, and taken up with hydrochloric acid, as already described. On filtering, the filter will retain a portion of the silica. The rest is recovered, after the removal of the stannous sulphide, by evaporating to dryness, taking up with hydrochloric acid, and filtering through the same filter. It is washed, dried, ignited, and weighed as silica. The filtrate from the silica is boiled with a little nitric acid and precipitated with ammonia. The precipitate is collected, washed, ignited, and weighed as ferric oxide and alumina (but it frequently contains oxide of titanium). When the last is present it is determined by fusing with bisulphate of potash and extracting with cold water. The solution is nearly neutralised with ammonia, charged with sulphurous acid, and boiled. The precipitate is collected, washed, dried, ignited, and weighed as oxide of titanium, TiO2. The difference between this weight and that of the combined oxides gives the ferric oxide and alumina. The filtrate from the mixed oxides is examined for lime and magnesia.
Sulphur.—Rub up 5 grams of the ore with 5 grams of nitre, transfer to a porcelain dish, and fuse over a Bunsen burner for fifteen minutes. When cold, extract with water, and determine the sulphur volumetrically with standard barium chloride. The sulphur may be present as sulphide or sulphate.
Arsenic.—Take 5 grams, and evaporate with nitric acid; dilute, add ammonia, pass sulphuretted hydrogen, and filter.
To the filtrate add “ magnesia mixture.” Collect the precipitate, ignite with nitric acid, and weigh as magnesic pyrarsenate.
The following may be taken as an example of the composition of an impure black tin :—

Examination of Hardhead.—In the smelting of tin ores a quantity of speise, known as “hardhead,” is produced. It is essentially an arsenide of iron, carrying a considerable quantity of tin. Much of this last is present in the form of small buttons of metal distributed through the mass. The buttons can be seen on careful inspection, and become evident on powdering.
In assaying the substance, a variation in the usual method of sampling is required, because of the quantity of metal present which cannot be powdered. After powdering as finely as possible, the coarse particles are sifted off and weighed. The weight of the powder is also taken. The method of working is best illustrated by an example. A sample of hardhead weighed 155.1 grams, and gave 21.0 grams of coarse particles, equivalent to 13.5 per cent, of the whole. The fine portion weighed 134 grams, which is equivalent to 86.5 per cent.
Thirteen and a half grams of the coarse material were dissolved in aqua regia, and diluted with water to 1 litre. Ten c.c. of this contain 0.135 gram of the metallic portion, which is the amount contained in 1 gram of the original hardhead. If, in a determination, 1 gram of the substance is wanted, weigh up 0.865 gram of the powdered portion, and add to it 10 c.c. of the solution. It will be seen that these together make up 1 gram of the original sample. The solution of the metallic portion must be saved until the analysis is finished.
Tin and Copper
Weigh up the portion of the powdered stuff equivalent to 1 gram of the sample. Transfer to a flask, and cover with 10 c.c. of the solution of the metallic portion and 10 c.c. of aqua regia. Boil gently till oxidation is complete and the nitric acid for the greater part driven off. Dilute to 100 c.c. with water, and pass sulphuretted hydrogen for some time. Filter, wash with hot water, and rinse through the funnel back into the flask. Digest with yellow sodium sulphide until only a light, flocculent, black precipitate is left. Filter this on; wash with hot water, dry, calcine, treat with a little nitric acid, ignite, and weigh as copper oxide, CuO. The weight multiplied by 0.7983 gives the weight of copper.
The filtrate containing the tin is rendered acid with hydrochloric acid, and filtered. The precipitate is rinsed into a half-pint beaker, covered with 20 c.c. of hydrochloric acid, and boiled down to about 20 c.c. The solution is filtered off from the sulphur and sulphide of arsenic, which, after washing with hot water, is transferred to a flask labelled “ arsenic.” A strip of sheet zinc (2 in. by 1 in.) is placed in the solution. The evolution of hydrogen should be brisk. In five or ten minutes decant off a few c.c. of the liquid, and test with sulphuretted hydrogen for tin. If no yellowish precipitate is formed, decant off the rest of the liquid, and wash the precipitated metal with hot water two or three times by decantation. The metal should be in a lump; if there are any floating particles they must be made to sink by compression with a glass rod. Transfer the washed metal to an evaporating dish 3 or 4 in. across, and cover with a few c.c. of hot water. Add nitric acid drop by drop till the tin is completely attacked. Evaporate nearly to dryness, and add a drop or two more of nitric acid and 20 c.c. of water. Boil and filter. Wash with hot water, dry, ignite, and weigh as stannic oxide, SnO2. Calculate to metallic tin by multiplying by 0.7867.
The filtrate from the first treatment with sulphuretted hydrogen will probably no longer smell of the gas. Warm and pass the gas for a few minutes longer. Filter off any precipitate of sulphide of arsenic, and transfer it to the flask for “arsenic.” Boil the filtrate (ignoring any signs of a further precipitation of arsenic) with a few c.c. of nitric acid, and separate the iron as basic acetate. Wash ; reserve the filtrate for cobalt.
Iron
Rinse back the “ basic acetate,” precipitate into the flask, add ammonia, dilute with water to about 100 c.c., and pass sulphuretted hydrogen for a few minutes. Filter, and wash with hot water. Collect the filtrate in the flask labelled “arsenic.” Boil the precipitate with dilute sulphuric acid, filter, and titrate the filtrate with the permanganate of potassium solution after boiling off the sulphuretted hydrogen. Report the result as iron. The sulphuric acid will not effect complete solution, a light black residue will remain, chiefly sulphur; this must be rinsed into the filtrate from the acetate separation. It contains cobalt.
Cobalt
The filtrate from the acetate separation will have a pink colour. Render it ammoniacal and pass sulphuretted hydrogen. Collect the precipitate on a filter, dry, and ignite. Dissolve in hydrochloric acid, and evaporate nearly to dryness with an excess of nitric acid. Dilute with 10 or 20 c.c. of water and add potash solution in slight excess. Add acetic acid until the solution is acid and the precipitate is quite dissolved. Add 20 or 30 c.c. of a strong solution of potassium nitrite, and determine the cobalt, as described on pp. 254, 256. Boil the filtrate from the cobalt, precipitate with hydrochloric acid, render ammoniacal, and test for zinc, nickel, and manganese.
The remainder of the tin will be contained in the flask labelled “arsenic.” Acidify with hydrochloric acid and filter. Rinse into a beaker, and evaporate to a small bulk with 10 c.c. of nitric acid. Dilute and filter. Dry the precipitate, consisting of stannic arsenate (2SnO2.As2O5), ignite, and weigh. Calculate the tin it
contains by multiplying by 0.4453, and add to that already found.
Arsenic
This is determined in a separate portion. Weigh up a portion of the powder equivalent to 1 gram of the hardhead, place in a pint flask, and boil with 10 c.c. of nitric acid. When action has ceased add 10 c.c. of the solution of the metallic portion and then hydrochloric acid (a few drops at a time) till solution is complete. Warm gently in dissolving, but do not boil. Dilute to about 100 c.c., render alkaline with ammonia, and add 20 c.c. of yellow ammonium sulphide. Digest at a gentle heat for about thirty minutes, filter, and wash. Add 50 c.c. of magnesia mixture, shake well, allow to stand for an hour, filter, and wash with dilute ammonia. The precipitate is dissolved and then titrated with uranium acetate, or it is evaporated with nitric acid, ignited, and weighed as pyrarsenate of magnesia. Calculate the result to arsenic, As.
Sulphur
Weigh up a portion of the powder equivalent to 2 or 3 grams of the hardhead. Rub up in a mortar with 5 grams of nitre and fuse in a porcelain dish for ten minutes. Extract with water, add 20 or 30 c.c. (as the case may be) of the solution of the “ metallics.” Add 10 grams of sodic acetate, and ferric
chloride until the precipitate turns brown ; dilute with water to half a litre, boil, and titrate with standard baric chloride, as described under Sulphur. Report as sulphur.
A sample of hardhead examined in this way gave—

Examination of Tin Slags
In tin smelting works the term “ slag ” is applied to the unfused portion of the charge. It is made up of unburnt anthracite and small lumps of slag proper together with some buttons of metallic tin. This is rarely, if ever, assayed. The slag proper (or, as it is generally called, “ glass ”) is a silicate of iron, alumina, and lime, containing from 3 to 7 per cent, of tin. It is thus examined :— The sample after bruising on an iron plate, is reduced to a very fine powder by grinding in an agate mortar. In this state it is in most cases readily decomposed by hydrochloric acid.
Determination of Tin
Where the percentage of tin only is required, take 2 grams of the powdered slag and well mix with it 20 c.c. of hydrochloric acid, and heat to boiling. Add 1 c.c. of nitric acid, allow to stand for fifteen minutes, dilute with water, and filter. Pass a rapid current of sulphuretted hydrogen for some time. Allow to settle, and filter. The precipitate, after washing with hot water, is dried, and gently calcined until the greater part of the sulphur is burnt off. It is then strongly ignited in the muffle (or over the blowpipe) with the addition of a small lump of ammonic carbonate. The residue is weighed as stannic oxide (SnO2); and is calculated to metallic tin by multiplying by 0.787. The percentage on the slag is calculated in the usual way.
The tin is always best determined in the examination of slags by a separate assay carried out in this way. The determination of the other constituents is thus made:—
Silica
Take 2 grams of the powdered slag and cover them, in a small evaporating dish, with 20 c.c. of hydrochloric acid ; mix well by stirring with a glass rod; and evaporate to dryness. If (as is generally the case) tungsten is present the solution will be blue. Take up with 20 c.c. of hydrochloric acid. Add 1 c.c. of nitric acid; and reduce by boiling to about half the bulk. Add about 20 c.c. of water, boil, and filter. Wash the residue with hot dilute hydrochloric acid. It consists of silica with the tungstic acid. Wash it back into the dish; and digest with 5 or 10 c.c. of a cold solution of ammonic carbonate. Filter; and collect the filtrate and washings in a weighed porcelain dish. Dry the residue, ignite strongly, and weigh as silica, SiO2. In certain exceptional cases this may contain some unaltered cassiterite, which is easily recognised by its appearance.
