Computing Cyanide Consumption of a laboratory leach test may be done as in the following example: Ore taken, 250 grams. Ratio of solution to ore, 3:1 = 750 cc: 250 grams. Cyanide strength, 0.3% KCN.
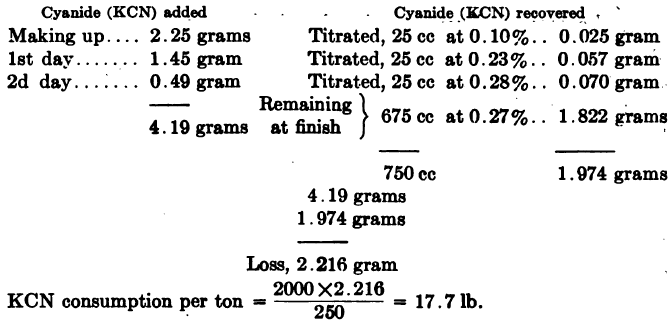
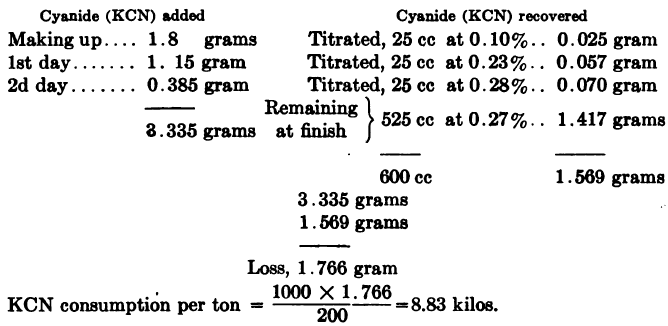
Or when working on the metric system,
Ore taken 200 grams,
ratio of solution to ore 3:1 = 600 cc: 200 grams,
cyanide strength 0.3% KCN
It should be noted that cyanide consumption as figured above represents chemical consumption only. In practice there will also be a mechanical loss of cyanide in residues, and also a loss to some extent in precipitation, if the zinc process be used. The mechanical loss in residues will depend on the strength of solution used, and also on whether the slime residue is dewatered by a filter or by decantation.
In the case of silver ores the chemical consumption indicated in a bottle test usually corresponds very closely with the actual total consumption to be expected in practice, and for this reason; an important factor in the cyanide consumption is the amount that combines with the silver: on precipitation this silver is replaced by zinc, and this zinc solution on coming in contact with the new lime added in the mill is partly dissociated and some free or available cyanide liberated. The amount of such cyanide thus recovered (a phenomenon which is not apparent in the ordinary laboratory test) comes near to offsetting the mechanical loss of cyanide in the filter residues.
Tests made as described will be found in most cases to approach very closely to working conditions, and to give results concordant with large-scale figures. A variety of combinations will suggest themselves to the operator. Assuming the ore to be “ all-slimed ” he will need to ascertain, among other things, the best ratio of solution to ore, the best and most economical cyanide strength, and the time of treatment necessary; and a series of bottle charges may be made up and carried through to elucidate each point separately.
The figure for the extraction obtained on such tests is usually based on the difference between the assays of the head and the residue, and this seems to be a perfectly proper procedure, since both head and residue sampling is completely under control. Some experimenters make a point of checking the extraction, calculated as above, by an assay of the solution, but on these small-scale bottle tests the fact of a portion of the solution being withdrawn each day for titration complicates the operation and renders the result less reliable than the figures based on the ore and residue assays.
High Cyanide Consumption
If experiments on any ore show an abnormally heavy consumption of cyanide (and assuming, of course, that protective alkali has been present in excess the whole time), it is advisable to try to ascertain the cause of the loss. By testing the solution after contact with the ore, for zinc, copper, silver, sulphocyanate, and ferrocyanide, it is often possible to account for nearly the whole amount consumed. Should there remain a considerable proportion unaccounted for it may be due to the presence and dissolution of some metal or metals other than those mentioned, or it may be due to oxidation by the use of excessive aeration, or occasionally to the presence in the pulp of organic substances.
It is only possible here to give an outline of the more common methods of preliminary experiment, and to suggest lines of investigation. Special conditions will call for variation and increase of detail, which each operator will be able to develop according to his needs.
Consumption of Cyanide – Feldtman’s Test
Having ascertained the amount of gold extracted, it is necessary, from a commercial point of view, to ascertain the amount of cyanide required for this extraction. The method of procedure is as follows :—50 gms. ore + 100 c.cs. .5% KCN solution are placed in a 300 c.c. flask and agitated every few minutes for a period of fifteen minutes. Filter off some of the solution through glass wool and estimate the available KCN by AgNO3. The difference between this and .5% gives the consumption of KCN. If 2000 lbs. ore require 1200 lbs. .5% solution, and out of this .3% is consumed, the consumption is 3.6 lbs. per ton of 2000 lbs. As all this cyanide is not consumed in dissolving gold, the chemist then proceeds to find the cause of the consumption.
(a) Test for acidity, which may be either ‘soluble’ or ‘latent.’
To determine ‘soluble’ acidity, agitate 10 gms. ore with 50 c.cs. water; filter and wash till free from acid. Titrate with N/10 NaHO, indicator litmus. Note the number of c.cs. soda used.
To determine ‘latent’ acidity, add to the residue in a flask a measured excess of N/10 soda (NaHO). Agitate for five minutes; let stand five minutes. Filter and thoroughly wash. Titrate solution with N/10 H2SO4. The difference between the c.cs. N/10 H2SO4 and the c.cs. N/10 NaOH give the amount of N/10 NaOH neutralised by the latent acidity.
Soluble + latent acidity = total acidity. Calculate the amount of CaO necessary to neutralise the total acidity. Proceed with another shaking test, using the same quantities as before plus about 50% more than this theoretical quantity of lime. Besides neutralising acidity, the student should note that CaO breaks up certain double cyanides of metal and potassium, setting free potassium cyanide. If the consumption of cyanide is still excessive, proceed to test for the following (amongst other) obnoxious substances (cyanicides), soluble iron salts, soluble sulphates, copper and antimony compounds.
At present the student need only apply qualitative tests for these substances.
If tellurium be present, grind a sample of the ore to pass through a 100 mesh sieve, and carefully roast ‘dead.’ Then proceed with fresh extraction and consumption tests.
