WASHING THE PRECIPITATE: A precipitate may be washed directly on the filter, or it may be washed partly by decantation and partly on the filter. If by decantation, the precipitate is allowed to settle, and the supernatant liquid is poured on the filter. Wash water is added to the precipitate, and after settling, the decantation is repeated a few times, and finally the precipitate is transferred to the paper or Gooch crucible.
Whatever method of washing be used, it must be thorough; and that this may be so, both the precipitate and the paper or asbestos must be washed free from all traces of the original solution. This must be ascertained by careful tests ; guesswork on the ‘ good enough ’ principle is the sure road to inaccuracy, and must never be permitted. It may appear that the chemist sometimes guesses at the completeness of a washing. This is not so, as when he does not apply a test he knows from experience gained in many analyses that, say, four washings serve to remove certain salts. The student must likewise gain experience by continually applying tests; and it is only in routine work, where hundreds of analyses of a kind are performed, that it is at all justifiable to omit tests.
For practice, let the student place a paper in the funnel and thoroughly moisten it with E. H2SO4 ; then let him wash it by a jet of hot water, applied first at the upper edge and then downwards to the point, till the paper is over half filled with water. Let this drain. Repeat the washing three times. Dry the funnel and paper in the air bath at 110° C. Then cautiously increase the temperature. If any H2SO4 remains in the paper a black stain appears. This forms a good check on his manipulation. The general failing of students is to neglect the top edge of the paper. The results can also be checked in a repetition of the experiment by testing every now and then about 1 c.c. of the washings caught in a test tube with a little BaCl2 solution.
Unless otherwise directed, hot water should be used for washing; and as a rule, when using filter paper, each washing should drain through before applying another. In this way a smaller quantity of water will do the required work. This is of importance, as, when the filtrate is to be further treated, a large bulk is objectionable. When the Gooch method is used, the washing water is added before the crucible is drained, a little liquid being kept above the filter till the final wash, when the crucible is drained.
Particles of precipitate adhering to a beaker may be removed by rubbing with a piece of rubber tubing on the end of a glass rod.
In testing the completeness of a washing, the most delicate qualitative tests must be applied. The student should now be in a position to apply these tests intelligently. If the filtrate is reserved for further treatment, tests should not be applied till about the third wash, as a certain quantity of the filtrate is thus lost.
If a chloride and a nitrate be present in the solution it will be safe to test the washings for chlorine alone, and assume that if the chloride is washed out, the nitrate has also been removed, as its solubility is not far removed from that of the chloride. This assumption is further justified, in that the qualitative tests for chlorides arts more delicate than those for nitrates.
When applying the jet from the wash bottle a gentle pressure from the mouth on the water in the bottle must be maintained, and care must be taken that the precipitate is not splashed up the sides of the glass funnel, or that the layer of asbestos in the Gooch crucible is not disturbed. The jet should not be directed on the precipitate till a steady stream has been obtained. A small stop valve ground into the bottom of the delivery tube, as shown in fig. 54, may not be beyond the student’s capabilities in glass working, and serves to maintain a column of water in the delivery tube, and prevent the first portion coming through the jet from splashing the precipitate.
DRYING & IGNITING THE PRECIPITATE
After the precipitate has been separated from the solution, it has in some cases to be dried and then weighed; in others, it has to be dried and ignited before weighing. The operations of drying and ignition and the necessary apparatus will now be described.
The Apparatus required
Precipitates are generally dried in water or air ovens. When the drying temperature is not to exceed 100° C. the water oven is used, and when over 100° C. the air oven. The actual temperature of the precipitate in the water oven rarely, if ever, reaches 100° C. under normal conditions; and when instructed to dry a precipitate in the water oven at 100° C., it will be sufficient to heat the water to the boiling point and transfer the precipitate to the oven. If specially desired, the boiling point of the liquid in the jacket may be raised by adding a little salt to the water.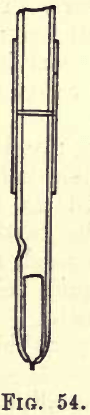
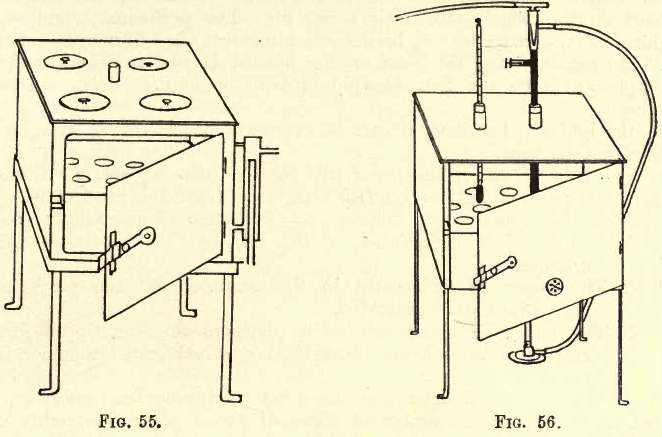
The water oven itself is shown in fig. 55, and is made of copper. It consists of a chamber with one or more shelves perforated with holes. This chamber is provided with a door at the front, and on all sides, except the front, is surrounded with water contained in an outer chamber or jacket. The top of the oven shown is adapted for evaporations, and at the side is a constant level feed for the water supply. A cork, carrying a thermometer to indicate the temperature, is inserted in an opening provided on the top of the oven. The thermometer bulb should come well down to the centre of the inner chamber. The water oven may be connected with a condenser, and a supply of distilled water is thus economically procured.
When a precipitate is to be dried on a paper, the funnel and paper are transferred to the oven, the stem of the funnel being passed through one of the holes in the shelf, and the top of the funnel is loosely covered with a piece of filter paper to prevent contamination by dust, etc., or the filter may be removed from the funnel and set on its side on a large watch glass. The burner under the oven is lighted and heat applied till the precipitate is apparently dry and no more vapour is seen rising from its surface. The door is closed during drying, and is only opened for removal or inspection.
The operation of drying may also be carried out conveniently and quickly in the air oven, provided the temperature he carefully regulated. By a little experience the student will be able to control the temperature of the oven within a few degrees of the required point by adjusting the bunsen flame, but, for more accurate work, a gas regulator (so called Thermostat) should be fitted to the oven.
The oven is made of stout sheet copper, in the form of a cubical box with a door on the front. The door has a small shutter for admission of air. Inside is a shelf about 4 cm. from the bottom of the oven, and another shelf may be inserted, if required, higher up. The oven rests on a four-legged stand, and is heated by a bunsen burner. In the top of the oven are two holes, one for the thermometer and the other for the gas regulator. It is advisable to cut a V-shaped notch along the cork carrying the thermometer, as this permits the easy escape of vapour. The oven, with the thermometer and gas regulator in position, showing the connection to the gas supply, is seen in fig. 56.
The gas regulator is made of glass; the long thermometer-like tube and the first cross arm are filled with mercury. Tn this cross arm is a screw which can advance or retreat, and force the mercury up or down the tube. The gas is led through the regulator as shown, and when the temperature exceeds a certain point the mercury in the tube rising shuts off part of the gas and thus lowers the bunsen flame. By adjusting the screw and the quantity of mercury, the temperature may be kept constant at any desired point. For convenience, the student may regulate the temperature to 110° C. A little alteration of the screw inwards or outwards will set the regulator at any other temperatures required.
The thermometer bulb should come within an inch of the lower shelf if the substance to be dried is placed on that shelf. If a funnel and contents are to be dried a middle shelf should be used, the stem going through one of the holes in the shelf. When the temperature is of importance an article should not be set directly on the lower shelf, as it is usually somewhat hotter than indicated by the thermometer. A square of asbestos board is convenient when placed on the lower shelf, as substances may be dried upon it without danger of overheating.
Precipitates in the Gooch crucible are dried in the same way. Care should be taken that the shelves on which the crucible is placed are perfectly clean, and that the roof of the oven has no hanging scale which may fall into the precipitate.
After drying, the precipitate frequently has to be ignited, that is, heated to a dull or a bright red heat, either in the air or in a current of some gas such as hydrogen, carbon dioxide, coal gas, or oxygen.
When paper has been used for the filtration, this as well as the precipitate must be incinerated. The necessary apparatus will be shown in the following illustrations, and consists of a bunsen burner, an iron tripod about 20 cm. high, a pipe clay triangle for porcelain crucibles, and a platinum wire triangle for the platinum crucible, a glass stirring rod with rounded end (or a stout platinum wire), porcelain crucibles and lids (about 2.5 cm. in diameter by 2.5 cm. deep), and if possible a platinum crucible and lid (weight about 20 gms. to 30 gms., cost £3 to £4, 10s.), crucible tongs.
Methods of Incineration
When the reducing action of the carbon will have no action on the precipitate, the paper and precipitate are first dried and then are placed in the crucible, which is set in the triangle on the tripod. The crucible is tilted at an angle of about 45° and the bunsen flame is gently applied—at first by taking the burner in the hand and moving the flame across the bottom of the crucible. The paper takes fire and burns, leaving a black mass. The burner is now set under the crucible so that the flame strikes the bottom, and the heat is increased and the ignition continued, with occasional careful stirring by the glass rod or platinum wire, till all black particles disappear. The crucible is then removed with the crucible tongs and placed in the desiccator, the tongs being replaced on the bench with their points up. The preliminary ignition and weighing of the crucible will be described under “ The Estimation of Filter Ash.” In certain cases the heat of the bunsen is not sufficient, and must be supplemented by the foot blowpipe (blast) or ignition in a muffle (see Assaying).
In the ignition described, if care is exercised in preventing draughts, the lid need not be used. Where the carbon of the paper will have a reducing action on the precipitate, the paper must be ignited separately from the precipitate. This may be done in several ways, differing only in detail of manipulation:
- The paper is incinerated on the lid, and the precipitate in the crucible.
- The paper is incinerated in the crucible, and the precipitate is then added and incinerated.
- The paper is incinerated on a platinum wire (or tipped forceps), and then the ash and precipitate are incinerated together in the crucible.
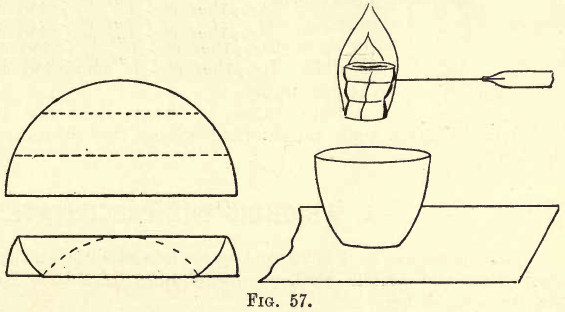
To prevent loss when separating the dried precipitate from the paper, the student should procure a number of pieces of glazed paper (preferably black) about 15 cm. X 22 cm. A piece of this paper is placed on the clean bench, and by gentle squeezing and shaking the precipitate is, for example in the third method mentioned, transferred to the crucible which has been placed on the glazed paper. Any precipitate falling outside the crucible is caught on the glazed paper. The filter paper is opened out, and by gently rubbing the interior surfaces against one another most of the remainder of the precipitate is removed. Fold up the paper and wrap round it a few turns of a platinum wire mounted in a glass tube or rod. Take the rod in the right hand so that the paper is about two inches above the crucible, and in the left hand take a bunsen burner and kindle the paper, as shown in fig. 57. When the paper ignites, remove the bunsen flame, and the charred remains glow till the carbon is all burnt away. By a gentle tap the skeleton of ash falls into the crucible. Any particles of precipitate or ash on the glazed paper are now transferred to the crucible, which is placed on another piece of glazed paper, the former piece being doubled scoop-like, and the particles brushed down with a small camel’s-hair brush, care being taken that no small particles are left entangled in the hairs of the brush. The crucible and contents are then ignited over the bunsen or blast flame as may be required.
In the first and second methods mentioned the details are obvious, the precipitate in the second method being transferred to a piece of glazed paper, and covered with an inverted glass filter funnel whilst the ignition of the paper proceeds.
The superiority of the Gooch methods is now very evident. There is no separation of filter and precipitate; and after drying, the precipitate is ready for incineration. The cap is slipped on and the crucible set on the triangle and the ignition commenced at a low heat, gradually raising the temperature till the incineration is complete. If the porcelain Gooch crucible is not provided with a cap, care must be taken when incinerating that particles of the filter or precipitate do not get detached. As every additional handling increases the probability of error, it is seen that, besides mere saving of time, the Gooch method may justly claim a higher degree of accuracy than the paper method; and where precipitates are acted on by the carbon of the paper, or where a precipitate is to be dried and weighed without ignition, its use becomes more and more advisable. The student will find it convenient to provide himself with three or four porcelain Gooch crucibles and a platinum cap (made of thin foil) for ignition.
As the student frequently makes use of filter papers, he may now proceed to estimate the ash of the papers he intends using.
Take a porcelain or platinum crucible; place it in a triangle on a tripod, and gently heat up to a dull red. Keep it at this temperature for ten minutes. Remove the flame, and in two or three minutes transfer to the desiccator. When cool (in ten to fifteen minutes) quickly weigh the crucible, replace it in the desiccator and reweigh after five minutes. This is simply a check. If this weight is not the same as the previous one, the operation must be continued till two successive weighings do not differ by more than .0003 gm.
Take ten of the papers, fold them and incinerate them one by one on the platinum wire, as before directed, allowing the ash to drop into the crucible. When the ash of the ten papers has been transferred to the crucible, place it on the tripod and ignite at a red heat for ten minutes, carefully stirring the ash to make sure that no black particles of unconsumed carbon remain. Remove the flame, and after two or three minutes place the crucible in the desiccator, and when cool weigh as before. Ignite for another five minutes, and repeat the desiccation and weighing. Then assuming

∴ Weight of ash of one paper = .0090/10 gm = .0009 gm. And when one of these papers is incinerated with a precipitate, this weight must be deducted from the joint weight to obtain the true weight of the precipitate.
It is of importance that when weighing the crucible alone the preliminary incineration should be carried out as directed. A few minutes heating will not suffice, and half an hour or an hour will be too long. Again, if the precipitate is to be incinerated over the blast, the crucible in the preliminary ignition should be heated for about five minutes over the same blast. It is only by careful preliminary and after incineration, combined with systematic desiccation and weighing, that uniform results may be expected.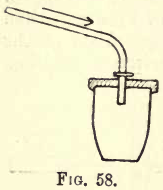
To save delay in weighing and gain by absorption of moisture from the air, place weights approximately lower than the probable weight of the crucible and precipitate on the pan. A little experience is here necessary, but the habit should be cultivated from the outset. With some precipitates ignition in a current of oxygen, coal gas, hydrogen or carbon dioxide may be advisable. Rose’s crucible (fig. 58) will be found serviceable for this purpose. The pipeclay tube is clamped on a retort or other stand, so that its nozzle projects through the lid into the crucible. Its other end is connected by rubber tubing to the gas supply.
Bulb tubes of hard glass are sometimes used for ignitions in gases such as chlorine, where the substance ignited is not to be weighed.
WEIGHING THE PRECIPITATE
This operation, as it is intimately connected with ignition and the use of the desiccator, and as the methods of weighing have been described, will only be briefly noticed here.
When selecting a crucible for a given precipitate, besides considering the material of the crucible and the nature of the precipitate, some thought must be given to the weight and volume of the precipitate and the size and weight of the crucible. If the student has two porcelain crucibles weighing 15. +and 6. + gms. respectively, he will select the smaller crucible if it will conveniently hold the precipitate, as there is less liability to error through condensation of moisture. The less the surface exposed the less the condensation, and with a large crucible and very small precipitate the percentage error thus introduced may be serious.
The precipitate is sometimes weighed (without ignition) on counterpoised papers (or on a weighed filter paper); but as paper is hygroscopic, this method should be replaced by the Gooch crucible. When this is not available, the two papers are dried at 100° C. and counterpoised on the balance pans, roughly by clipping, and then by a rider. Both papers should then be subjected to the same treatment, one paper being run as a blank beside the other containing the precipitate. The papers, after the usual washing, are dried, transferred to the desiccator and weighed, making allowance for the rider previously used, the difference in weight giving the weight of the precipitate.
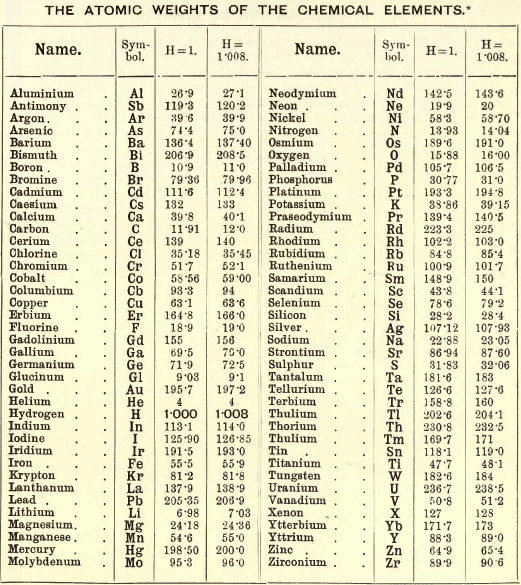
Table of Atomic Weights
The student will see that two values are placed opposite each element, one being based on the unit H = 1 and the other on the unit H = 1.008 or O = 16. In the course of his analytical work the student must take his values from one of these two columns, and from one only. Commencing on the assumption that H= 1, he will take all his values from the first column, but on the assumption that H= 1.008 (or O = 16), he will take them from the second column.
Some of the figures given in Parts II. and III. of this work do not agree with those in the table just given. When these sections were being written this table was not to hand, hence the discrepancies, for which the authors apologise, and ask the student to be good enough to refer to this table for all atomic weights, and substitute the figures in the table for those in the text. The student is advised to work from the second column.
https://www.911metallurgist.com/gravimetric-analysis-precipitation-reactions-examples
