There are two conditions generally prevailing upon the earth—those within atmospheric influence, tending towards oxidation, and those away from atmospheric influence, tending towards reduction. Practically all mineral substances from mines of any depth are in a reducing condition.
Since the cyanide process, in order to dissolve silver or gold, requires that the prevailing conditions under which it operates shall be oxidizing, and the materials usually acted upon being of a reducing character, it becomes necessary to supply oxygen to the solution carrying the cyanide. This oxygen is usually supplied through the medium of dissolved air in the solution, or through the medium of various chemical compounds, which upon combining with the solution or the ore give off a part of their oxygen.
Strange as it may seem, practically all mineral substances are partly soluble in water, especially water carrying alkali or cyanide. The greater the surface exposed and the liner the material is ground, the greater will be the rate of dissolving of the reducing-agents from the ore into the cyanide solution. In most cases, if the solution carrying the ore particles is agitated with air, the air will dissolve into the solution faster than will the reducing-agents; but in some cases the reducing-agents will dissolve more rapidly on account of easy solubility or greater surface exposed. It is a dissolving race between the oxygen from the air and the reducing-agents from the ore, and if the reducing-agents predominate, cyanide will not dissolve the gold from the ore. In many cases it will dissolve some of the gold, because in a mass of irregular shape some of the gold particles might be exposed upon the outside surface of a particle of rock; but if the solution had to penetrate through cracks, the side-walls of which were lined with reducing-agent- producing material, before the solution carrying oxygen could reach the gold it would have lost its oxidizing power. For this reason in many cases cyanide solutions will produce only a partial extraction of the gold or silver present.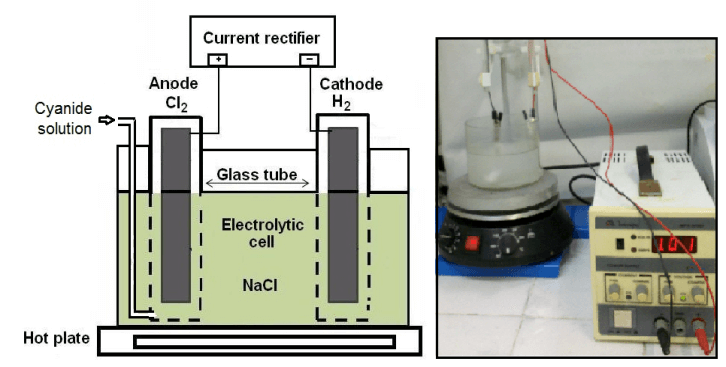
It occurred to me that since water is composed of hydrogen and oxygen, if it be decomposed by the electric current, the hydrogen would bubble away and the oxygen would be carried by the solution. This was tried in December, 1908, upon an ore carrying amorphous iron sulphides from which all the gold could not be dissolved by cyanide with simple air-agitation, no matter what the cyanide strength or how great the time, although the gold as revealed by the microscope was all metallic. The process was tried first in an inverted bottle with the bottom cut out, the air being forced in through a glass tube in the cork to agitate the pulp. Two lead plates were inserted in the agitated pulp at the top. These plates were about 4 in. long and 0.5 in. wide, and 1/16 in. thick. Through them was passed the current of an incandescent lamp, which being in series and burning dimly gave about 0.25 ampere of current. The results were excellent from the beginning. The value of the ore was $4 per ton. It was ground in a tube-mill so that 60 per cent, passed a 200-mesh screen. The value of the tailings, after 48 hr. agitation with air alone, was $1.25; but after agitation for 2.5 hr. with air and electrodes inserted in the pulp as described above, the value was reduced to $0.40. This typical result was verified perhaps a thousand times, with uniformly good results.
In testing our solutions, a 2-lb. solution of cyanide is test 10. The alkali is tested on the basis of ten points over and above the alkali due to the cyanide, test 10 being a 2-lb. solution of caustic soda. The reducing-agents were tested with a 1 per cent, solution of potassium permanganate, 1 cc. of which in 10 cc. of the solution, after acidulating, equals test 10, it being much easier to keep track of these solutions by simple numbers than by keeping the records in pounds per ton.
Numerous tests were made in order to determine a proper electrode. Lead was found to be the best material. Many other substances, such as carbon, worked very well, but with the alternating current, there being no consumption of the lead electrode, lead proved most satisfactory.
The following tests upon the working-solution show the effect of the different electrodes. All the tests were made at the same time and with the same solution, using the direct current.

There seems to be a regeneration of cyanide, and the process is certainly cheaper than any added oxidizer or even air-agitation of the solution.
We found by numerous experiments that the alternating current was as good as the direct current, and had the additional advantage of giving no deposit on the electrodes at lower current-density, and with lead there was no consumption of the electrodes even where the ore-pulp flowed over the electrodes. The way I explain this result is as follows:
Under the prevailing conditions certain electro-chemical actions take place by which the particles composing a molecule of a compound are resolved into the parts that the applied current-strength would resolve them into, and go into the solution on the one wave, and they do not re-combine on the returning current wave. In other words, dissociation takes place without being followed by re-combination. At any rate, no matter how the action is explained, it is carried on and works satisfactorily.
In electroplating, if the current is of low density the material deposited will be dense. If the current-density is increased, the material deposited will be spongy. If the current-density is still further increased, the material which should be deposited will he disengaged by the action of the gases, and practically no deposit will result, the material going into the solution in a more or less spongy condition. We found that with a very high current-density no deposit of gold or silver accumulated upon the lead electrodes with direct current. Some of the electrodes after being in use six months were scraped, and the scrapings assayed, and showed only a trace of gold and silver.
Electrolyzed solution seems to be especially effective when used in connection with lead acetate or litharge added in the tube-mill during grinding, The electrolyzed solution going to the tanks shows no sulphocyanides, whereas, before the batteries were put in use, the solution showed a large amount.
As finally used in practice in January, 1909, a battery, supplied with alternating current, was placed in the barren sump. This battery consisted of 18 plates in series, each plate 6 by 6 in., with 110 volts between the two. The plates consumed 15 amperes, and produced sufficient oxidizing effect, or whatever other effect it may be, to keep the solution in condition to treat daily 40 tons of this ore. These plates, made of 1/8-in. sheet lead, were built so as to form hollow rectangles in section, the rectangle being 6 in. high, 6 in. long, and 1.25 in. wide inside. The two ends were lapped at the top and holes punched. The plate was bolted to a paraffined plank 1 by 6 inch. in section; 18 of these plates were connected in series. The distance between any two plates was 1/8 in., and, of course, the current would travel principally across the 1/8 in. gap, instead of around the 1 3/8 in. gap, from plate to plate. Lead wires were used from the surface of the solution down to the plates. We ground the ore in the tube-mill so that 60 per cent, would pass a 200-mesh sieve. Previous to using the batteries in the sump, the extraction in the tube-mill was 20 per cent, during grinding; after the batteries were used, the extraction in the tube-mill was 75 per cent. The effect of the batteries seemed to build up in the solution gradually and to lose from the solution gradually when the operation of the batteries was discontinued.
During two months in the fall of 1910 the mill was working coarse ground, partly-oxidized ore carrying considerable sulphides. The water at the hydro-electric plant was low, and the use of the batteries was discontinued because the mill was driven with steam, and no arrangement had been made to supply alternating current from any but the hydro-electric plant. During this time the tailings on $4 ore went up to $1.25- per ton, and immediately after the rains gave sufficient water to drive the hydro-electric plant, the values in the tailings diminished until $0.20 per ton was reached on identically the same ore with the same head-values; moreover, the reducing-agents dropped from 16 to 4. The time occupied in getting the working solution up to this condition was two weeks. I consider that this process owes its value almost entirely to the presence of oxygen due to electrolysis, putting the solution ahead in the race with the reducing-agents and causing the gold and silver to dissolve in spite of the reducing-agents. However, it does not stop the reducing-agents from dissolving also, and although it produces solution of the gold in spite of the reducing-agents, it does not help precipitation, and if the reducing-agents are not decomposed by the batteries—and all of them are not—they build up in the solution rapidly to a point where zinc-shavings will not precipitate the gold.
Of course, in practice the cyanide solution contains reducing-agents of many kinds. The electrolytic action seems to reduce the influence of some, but not all of them. For example, I experimented on some highly-graphitic ore, and whether the normally-poor extraction was due entirely to the graphite or not, I do not know; but the solution, after electrolyzing, gave a very much better extraction than before electrolyzing. The action seems to decompose the sulphocyanides and the soluble sulphides, but not the alkaline sulphides and all of the many others always present.
A test on the eleetrolyzed solution 18 months after the batteries were installed showed:
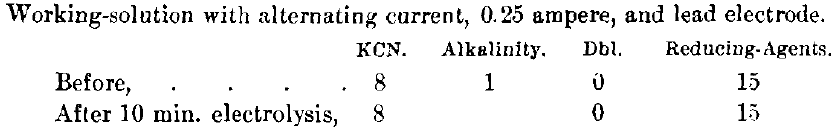
showing that the solution remained practically the same, or was electrolyzed as much as was necessary. However, testing some of this same solution further by placing a piece of gold leaf upon its surface and allowing it to float, the gold leaf was dissolved in 71 min. on the working-solution and in 50 min. on the re-electrolyzed solution, showing that the additional electrolysis, although it had no apparent effect on the solution, gave an increased dissolving-rate. Grease in the ore or on the surface of the barren sump seemed to dissolve very rapidly in the treated solution and slowly in the untreated solution. We tarred our tanks inside and coated them with black oil outside, and more or less grease was frequently floating upon the surface of the solution where this effect was noticed.
Since the installation of this process it has treated successfully at this plant 25,000 tons of ore of all kinds, oxidized, partly oxidixed, and sulphides. Previous to the use of the batteries, in treating sulphide ores, the average cyanide-consumption was 1 lb. per ton, in some months running as high as 1.1 lb. After the use of the batteries the average was 0.45 lb., running for some months as low as 0.23 lb. per ton of ore treated.
We tried using batteries in the agitated pulp and in the solution, and found the result to be just as good if the plates were inserted in the barren sump as if inserted in the agitated pulp. The original lead plates placed in the barren sump are still there and in operation. They cost about $4 to insert originally and were inspected after 26 months of practically continuous service, and are to-day just as good as when they were first put in use.
I have applied for no patents on this process and do not expect to, and any one is free to use it. It should be a cyanide-saver, an accelerator, and a general solution-purifier.
Electrolytic Oxygen in Cyanide Solutions, BY T. H. ALDRICH, JR.
