When reviewing Methods of Eliminating Copper from Gold Ores we see that several methods have been suggested to eliminate copper from ores prior to cyanidation. Preliminary extraction of the copper with sulphuric or sulphurous acids may be applicable to ores containing oxidized copper minerals such as malachite, azurite and chrysocolla but these acids have but little effect on sulphide copper minerals, many of which dissolve readily in cyanide solutions. In addition, oxidized ores very often contain appreciable amounts of carbonate minerals such as calcite and dolomite which dissolve rapidly in dilute acids. This leads to excessive consumption of acid.
 One method depends upon the property of cuprocyanide of potassium to dissolve copper in certain minerals. This cuprocyanide is obtained by heating the cupriferous ore with cyanide solution. When the cuprocyanide has dissolved its maximum of copper, part of the copper in the solution is precipitated electrolytically during which a partial regeneration of the cyanide is said to take place. The treated ore is then cyanided in the usual way and the gold recovered by electrolytic precipitation. Whether or not any precious metal was dissolved in the preliminary cyanide treatment was not stated.
One method depends upon the property of cuprocyanide of potassium to dissolve copper in certain minerals. This cuprocyanide is obtained by heating the cupriferous ore with cyanide solution. When the cuprocyanide has dissolved its maximum of copper, part of the copper in the solution is precipitated electrolytically during which a partial regeneration of the cyanide is said to take place. The treated ore is then cyanided in the usual way and the gold recovered by electrolytic precipitation. Whether or not any precious metal was dissolved in the preliminary cyanide treatment was not stated.
In another elimination method the copper mineral is dissolved out by means of ammonia after which the ore is cyanided as usual. The copper and ammonia are recovered from the preliminary leach solution by distillation, the copper remaining as an oxide precipitate. The success of this process will depend largely upon the relatively complete solubility in ammonia of those copper minerals which dissolve in cyanide.
The Hunt method-calls for the direct treatment of the ore with a solution of potassium cyanide to which ammonium hydroxide has been added. The gold is thus extracted together with some copper, and the metals are recovered by electrolytic precipitation, the gold, silver and copper falling to the bottom of the vats as a sludge. Zinc precipitation may also be used. The product is low grade.
A old-time metallurgist stated that in treating cupriferous gold and silver ores by the Hunt Process, the strength of ammonia in the solution is varied according to the copper content as well as to the combinations in which the copper is found. It was in use before for treating amalgamation tailings. This material contained a few pounds of copper per ton, present mostly as oxide. In its treatment, it was found necessary to use 8 lb. of caustic ammonia per ton of solution. The strength of the cyanide was 0.05%.
Another example was that of an ore treated in California, which contained cupriferous, pyrites and silicate of copper. The cyanide consumption was 7 to 8 lbs. per ton which using a 0. 15% cyanide solution. When 6 lbs. of ammonium chloride was added to each ton of solution together was sufficient lime to convert all of the ammonium chloride to the hydroxide, the consumption of cyanide was reduced to 1 lb. per ton of ore.
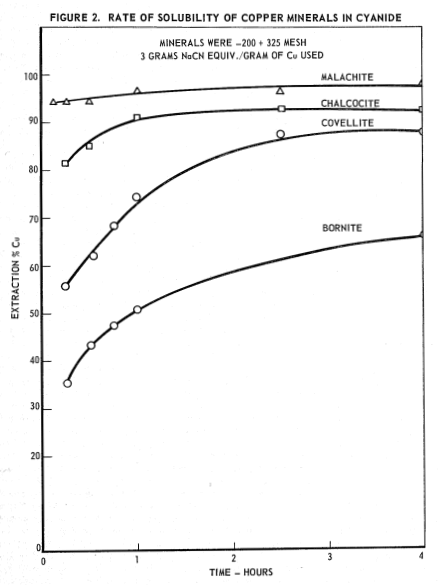
The use of cyanides in the extraction of gold and silver is well known. Such extractions employ concentrations of cyanide in the range of 0.02-0.25% sodium cyanide equivalent in leaching cycles of 24-48 hr and frequently longer. Copper minerals, even though minor components of precious metal ores, dissolve in these cyanide leaching solutions, consume cyanide, cause fouling of mill solutions, and thus interfere with the precipitation and recovery of gold.2,3,4 Here is a summary of laboratory investigations on the use of cyanide solutions as extractants for copper from various copper-bearing ore fractions. In view of the metal contents involved, it will be apparent that larger quantities of cyanide are required to extract copper than for the extraction of precious metals. In cyanide solutions of concentrations generally favorable to copper extraction, sulfide and oxide copper minerals were found to dissolve rapidly at room temperature and atmospheric pressure.
