The following remarks on the use of the fire assay fluxes and litharge may serve as an aid in arriving to a correct composition of charges for most cases:
Litharge or red lead is added in the proportion of one or two parts to two of ore; if too much litharge (lead oxide) is used the slags are not clean, as a slag containing lead may mean a loss of silver and gold. Whatever method is used, the amount of lead to be reduced should be from 25 to 60 grammes. Raw ores or regulus containing much sulphide of copper may be fused with from 4 to 6 A,T. of litharge to 1 A.T. of ore. In this case the other fluxes, except sand, may be omitted. Some assayers prefer to concentrate the copper as a regulus, and then to treat this over again; little more than the ordinary quantity of litharge is then used, and not much iron.
The amount of the charcoal added varies with the reducing power (percentage of ash) of the particular sample which is employed, as well as the degree of oxidation of the ore. In some highly basic oxidised ores as much as 3 grams of charcoal powder is required for 1 A.T, of ore, as the oxide must always be completely reduced to FeO, but generally from 1 to 1½ grams is enough. If there is much Fe2O3 in the ore (e.g., roasted pyrites) the slag is often rich, 3 or 4 per cent, of the gold being lost. The remedy usually adopted is to increase largely the quantity of soda carbonate, while sand must also be added to prevent the crucible from being perforated by the scouring action of the ferrous oxide. If ores contain much sulphur no charcoal is used, and nitre may even be added to burn off the excess of sulphur, otherwise, if the old practice of adding much litharge is followed, the amount of lead reduced may become too great. The addition of nitre is, however, now seldom made, as the pot is liable to boil over; with very large quantities of sulphides it is better to make a matte and treat the latter again.
Carbonate of soda is used to flux silica, while borax is valuable in basic ores to prevent corrosion of the crucible, and render the slag more liquid. The relative amounts required are judged from the appearance of the ore in the first place, and afterwards modified according to the success of the fusion. From 1 to 1½ A.T. of soda carbonate, and from ¼ to ½ A.T. of borax to 1 A.T. of ore are the amounts usually required. Even when the ore is entirely composed of silica, some borax is added. The most convenient form is borax glass.
Silica is used only for ores full of lime, baryta, compounds of the base metals, &c., or generally whenever the ore does not contain much quartz. It aids fusion in these cases, and protects the crucible from corrosion. From ¼ to ½ A.T. to 1 A.T. of ore is generally enough. Fluorspar is added to the charge when the ore contains sulphates of barium or calcium, and in the fusion of cupels. Like borax, it increases the fluidity of almost any charge, but it attacks the crucible, and care must be taken to avoid deficiency of silica when it is used. In general, it may be noted that for basic impurities an acid flux is used, and for an acid gangue a basic flux.
The ore is weighed accurately to .01 gramme unless very poor when a less exact approximation is sufficient. The charcoal is always weighed with great care ; the litharge is best measured by a ladle or shot measurer; the fluxes may also be measured out by a ladle. By practice the assayer becomes very rapid in measuring out reagents. The various ingredients are thoroughly mixed together on rubber cloth or in the crucible in which the fusion is to take place. Part of the borax is kept separate and used as a cover, being put on the top of the rest of the charge after transference to the crucible. Iron is added to the charge in the shape of large nails or hoop-iron, or even scrap, if sulphur or arsenic is present. Sulphur is thus kept out of the lead button.
The crucible is carefully annealed in the ashpit of the furnace before using. It is lowered into a hollow in the fuel of the furnace made by piling the coke round an old pot and then carefully withdrawing the 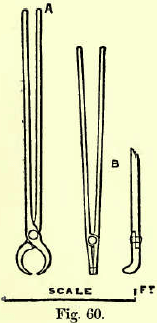 latter. The tongs most used are shown at B, Big. 60, A being those used when the fusion is performed in a muffle. The tire should be at a good red heat on charging-in, and care must be taken that no coke in the furnace is black. If the upper layer of coke is much colder than that below, the top of the charge in the crucible remains unmelted while the bottom begins to effervesce, and the crucible may froth over and part of its contents be lost. As the charge melts the fire is urged, and brisk effervescence occurs chiefly from the escape of carbonic acid from the soda carbonate as it unites with silica. After a lapse of from twenty to forty minutes the charge is in a state of tranquil fusion, with the exception perhaps of slight action round the sides or next the iron, if any is used. The crucible is then lifted out of the fire by the tongs, the nails withdrawn and any lead adhering to them shaken off into the pot. The pot may now be tapped on the floor to assist the lead to settle in the slag, allowed to cool and broken by a hammer to extract the lead button, or the charge may be at once poured into an iron mould. The mould must be cleaned, blackleaded and warmed before being used, and, after pouring, it is tapped on the bench to collect the lead at the bottom.
latter. The tongs most used are shown at B, Big. 60, A being those used when the fusion is performed in a muffle. The tire should be at a good red heat on charging-in, and care must be taken that no coke in the furnace is black. If the upper layer of coke is much colder than that below, the top of the charge in the crucible remains unmelted while the bottom begins to effervesce, and the crucible may froth over and part of its contents be lost. As the charge melts the fire is urged, and brisk effervescence occurs chiefly from the escape of carbonic acid from the soda carbonate as it unites with silica. After a lapse of from twenty to forty minutes the charge is in a state of tranquil fusion, with the exception perhaps of slight action round the sides or next the iron, if any is used. The crucible is then lifted out of the fire by the tongs, the nails withdrawn and any lead adhering to them shaken off into the pot. The pot may now be tapped on the floor to assist the lead to settle in the slag, allowed to cool and broken by a hammer to extract the lead button, or the charge may be at once poured into an iron mould. The mould must be cleaned, blackleaded and warmed before being used, and, after pouring, it is tapped on the bench to collect the lead at the bottom.
If the charge does not fuse completely, so that the slag is pasty or has lumps in it, it is advisable to recommence the assay, making such alterations in the charge as experience suggests.
When the mould is quite cold its contents are readily separated from it if the precautions mentioned above are taken, and the slag is detached front the lead button with a hammer on an anvil. The slag should be glassy and homogeneous; if it is streaked it is probable that the fusion has not been perfect. It is green and transparent if the ore is nearly pure quartz ; black and opaque if much iron is present; but is red if the ore contains, much copper.
The lead button should be soft and malleable. If it is hard or brittle it may contain sulphur, arsenic, antimony, copper. Sulphur and arsenic are kept from entering the lead by the addition of iron, and then form with the iron a regulus or speiss, which separates as hard blackish-grey or white layers found just above the lead. They are richer than the slag, and may often yield appreciable quantities of gold on further treatment by scorification or roasting and fusion. Antimony makes the lead hard, white, brittle and sonorous ; it can be removed by adding nitre to the fusion charge, but, according to Rivot, it is not a source of loss if forming less than 1 per cent, of the lead button (see under Cupellation below). The lead must be completely freed from the slag, very small quantities of the latter interfering seriously with the cupellation, forming a scoria and occasioning loss.
On the subject of fusing gold ores, Dr. E, J, Ball, who was formerly the Instructor in Assaying at the Royal School of Mines, London, wrote:
“ I find a very good plan in assaying a gold (or a silver) ore to be as follows, noting the points:
- That the main aim at the beginning of the assay is to produce an intimate contact between the molten reduced lead and the gold freed by the original grinding of the ore.
- That when this has been effected, the particles of ground-up ore must be, as it were, ‘atomically’ ground-up by solution, in order to liberate enclosed particles of gold.
“ The first of these stages should not be accompanied by a fusion of the charge, because immediately that happens the lead sinks to the bottom of the crucible, and is at once removed from the possibility of contact with the gold floating about in the charge. To bring about this contact in the second stage, the bath must be as thin-fluid as possible, and convection currents should be induced by irregular beating of the crucible, in the hope that, in the course of one of their gyrations, the particles of’ gold liberated by the solution of the quartz particles may strike against the surface of the molten lead at the bottom of the crucible.”
“ The maximum quantity of lead which the ordinary cupels in use at the Royal School of Mines will take is about 450 grains. I therefore recommend (when treating fairly pure quartz, containing say 1 oz. of gold per ton) that the charge should be made up as follows :—

The charcoal and red lead are first mixed together, and the ore is then carefully incorporated with them. Then 250 grains of sodium carbonate are roughly stirred in, so as to prevent the formation of a sort of sand-bottom, which would not dissolve in stage 2.
“ The charge is then maintained at as high a temperature as possible, actual fusion being avoided for fifteen minutes, when another 1,000 or 1,200 grains of sodium carbonate are charged in little by little, and the temperature raised to about 950°. At this stage, any necessary addition may be made in order to make the bath fluid, borax for instance, but borax seems to give low results if added at the beginning of the assay. I always add a piece of hoop iron to help to decompose any lead silicate or sulphide.
“ I never recommend the use of salt, but sometimes when, a large excess of sodium carbonate has been added, the ‘boil’ at the end seems never likely to stop, owing to the action of the acid crucible on the basic charge. In that case, a little salt stirred into the bath seems to volatilise, prevent the contact of the charge with the walls of the crucible for a moment or two, and to quiet the bath, enabling the charge to be poured properly.”
It is worthy of note that Dr. Ball reduces practically all the lead oxide, leaving none in the slag. Mr. H. C. Jenkins, the present Instructor in Assaying at the Royal School of Mines, while retaining most of the methods mentioned above, in teaching his students, recommends a larger addition of lead oxide, so that some is left in the slag. He never adds less than 40 grams of red lead to the charge, and aims at reducing from 28 to 30 grammes of lead. He recommends roasting highly pyritic ores (see below) and the use of nitre for antimonial ores only. He also advises his students to cover the crucible after fusion is complete. As giving examples of the charges which he employs, the following may be taken as typical:

with hoop iron ad libitum.
Roasting before Fusion.—Ores containing large quantities of sulphur, arsenic or antimony may often be roasted with advantage as a preliminary to fusion. Roasting is effected in shallow circular clay dishes, in a muffle, or in the crucibles in which the fusion is afterwards performed. The temperature must be kept low at first and the ore frequently stirred with an iron wire or spatula, to prevent fritting, and to expose fresh surfaces to the air. The roasting takes place in two stages : at first, sulphur dioxide, arsenious oxide (As2O3) and antimonious oxide (Sb2O3) are formed and volatilised, the sulphur burning with a blue flame. The formation of lumps is most to be feared during the first few minutes of the operation, and can scarcely be prevented if much sulphide of antimony is present ; in this case an equal weight of pure silver sand is mixed with the crushed ore before charging it into the muffle.
After a time the blue flame disappears, the odour become less strong, and sulphates, arseniates and antimonites form. By raising the temperature sulphates are decomposed, but arseniates and antimonites are stable at high temperatures and cause loss of silver in the fusion. To prevent their formation the ore should be roasted in a coke furnace, starting to heat it very gradually and admitting a limited supply of air. In all cases the roasting is nearly complete when the glow caused by stirring is shown only by a few specks of ore ; the temperature may then be raised to a strong red heat without danger of fusion. The operation is complete when the ore remains of a uniform colour on stirring. The fusion of roasted ores requires less lead than raw ores and more charcoal powder, the amount of the latter needed being something as much as 3 grammes per A.T. of ore. In general, roasting is to be deprecated, owing to the unavoidable loss by dusting. The results are usually lower than those obtained by fusion with the formation of mattes.
Aaron’s Fire Assay Flux Formula
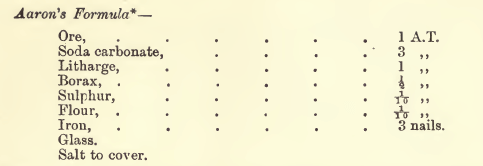
“Melt and leave in a hot fire about twenty minutes after fusion.” When ores contain only small quantities of base metals the following flux formula is recommended by:
Brown & Griffiths Fire Assay Flux Recipe

Percy’s Fire Assay Flux Formula
for ores containing only small proportions of base metals:


