Flotation, in its latest phase, is a process of concentrating ores by frothing. When crushed ore, previously mixed with water and a relatively minute addition of oil, is agitated violently in the presence of air, a froth is formed. This froth, rising to the surface of the liquid mixture, is laden with sulphides or other metallic particles, while the earthy material, or gangue, subsides to the bottom. The froth is “thick, coherent, and persistent.”
The formation of this froth depends upon a number of physical causes, of which the buoyancy of oil is the one most generally associated with the flotation process. Surface tension, however, is the phenomenon to be considered first. Then viscosity.
Every millman has had occasion to notice how sulphides are carried on the surface of wash-water in a stamp-mill; for example, when water is passed over the dry surface of an amalgamating-table or a vanner-belt. The metallic particles are dry and to their surfaces is attached a film of air that buoys them on the water. To a similar cause is due the loss of ‘float gold’ in tailing. Most of us learned early that grease of any kind was bad for amalgamation. It ‘sickens’ the quicksilver, coating the globules so that they do not coalesce but remain in a ‘floured’ or minutely globular condition. This may account for the loss of quicksilver, but the further loss of gold must be imputed to the fact that the fine scaly bright gold attaches itself readily to the oiled spheres of mercury and is carried with them into the creek.
The surface of any liquid behaves as if it had a film or elastic skin. To this fact is due the variation in the maximum size of drops of different liquids. As the drop enlarges, the strength of this skin is exceeded; then the drop breaks and the liquid falls. When an iron ring is dipped into a solution of soap, it will be seen, on taking it out, that a film of the liquid stretches across the ring. If a small loop of cotton, previously moistened with the solution, is placed on the film left on the ring, this loop can be made to assume, and retain, any form, such as is shown at A in Fig. 24. If, however, the film within the loop is broken, the loop immediately assumes the circular form, shown at B; and if it is now deformed in any way, on being released it springs hack at once to a circle.
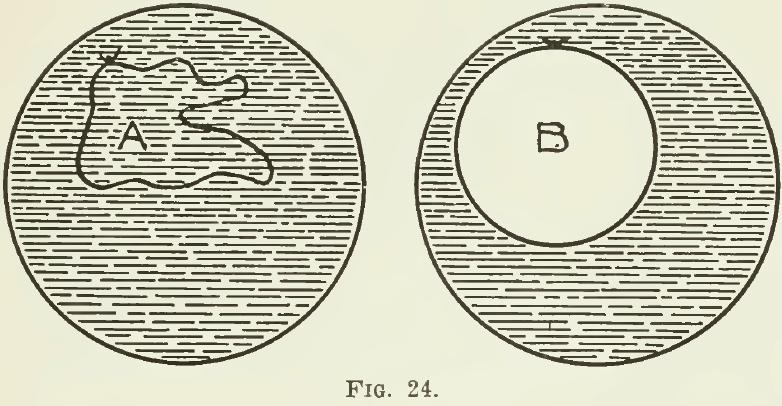
These phenomena indicate that the particles at the surface of a liquid have a greater coherence than the particles in the interior of the liquid. The force that does this is surface tension. The experiment with the ring and the loop, for example, is explained by the fact that, in the first place, “the surface tension of the liquid acts equally on both sides of the cotton, but when the film inside the loop is broken, the surface tension only acts on one side, and hence draws the loop out into a circle. ”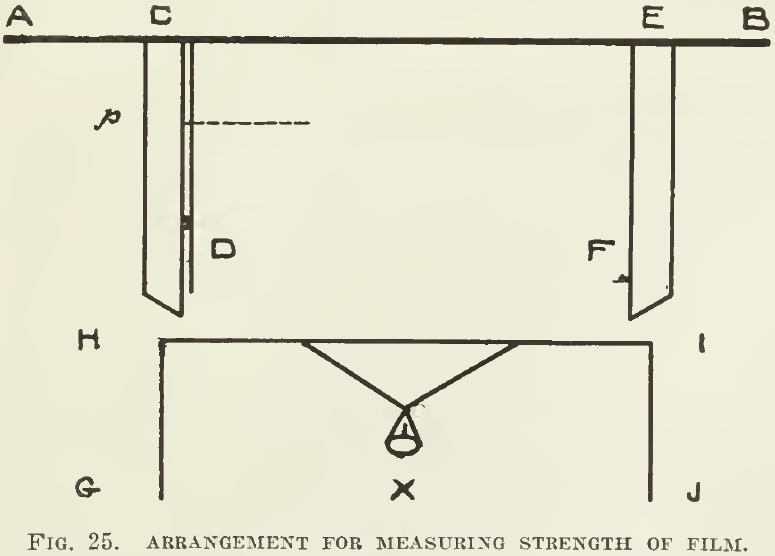
Surface tension can he measured. A framework (Fig. 25) consisting of a transverse bar A B, and two grooved slips C D and E F, will allow the piece of wire G H I J to slip freely up and down. The wire H I is pushed against A B and a quantity of the liquid is applied between them. The little pan X is loaded with sand until the wire H I is pulled from A B. The minimum force required to do this is mg, the weight of m grams. This weight suspended on the film equals the tension of the film on the wire. If the film stretches until the wire H I is at p, then the film has an area C E.Cp. The total weight mg is distributed over the breadth C E; whence, if T represents the superficial tension across the unit of length C E, then mg =T.C E or T = mg/CE
Thus the force of surface tension between water and air has been determined ; it is 3½ grams per linear inch or 81 dynes per centimetre, which being interpreted means that 40 grains would be supported by a film one foot long.
The surface tension of various liquids is as follows:
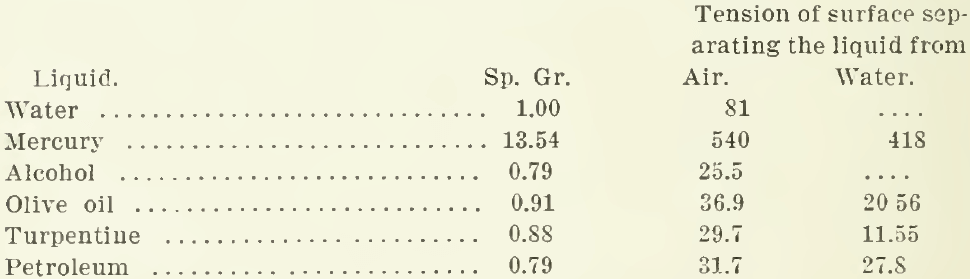
These are given in dynes per centimetre as determined by Quincke, and recorded in the Encyclopaedia Britannica. However, a liquid has another characteristic that must not be overlooked, namely, viscosity or resistance to flow. This gives toughness to the superficial film. Water-spiders will run over the surface of a pool like boys on skates over thin ice. The spider’s feet do not break through, although each tread makes a dimple on the surface. H. H. Dixon actually measured the pressure exerted by the spider’s feet on the water. He photographed the shadow of the dimple and then mounted one of the spider’s feet on a delicate balance and made it press on the water until it made a dimple of the same depth as that previously observed.
The next phase of the subject is illustrated by the familiar experiment with a greased needle. If you place an ordinary needle, say, a lace needle suitable for use with No. 80 thread, on the surface of a bowl of water, it sinks at once to the bottom, in obedience to the law of gravity. If, however, you pass the needle through your hair, so that it becomes greased, it will float on the water. Why the difference of behavior? In its ordinary state the needle has a film of air attached to it. That film, being loosely held, is readily displaced by the water, so that the needle becomes wetted, that is, its weight causes it to break through the elastic skin constituting the surface of the water. On the other hand, when the needle is greased, the film of air around it is displaced by a film of oil, which is firmly held, because lustrous metallic surfaces have a selective adhesion for oil. Moreover, gases have a marked adhesiveness for oils, so that air adheres readily to the film of oil on the needle. On account of this envelope of oil and air, the needle is not wetted, that is, it fails to rupture the surface. The needle lies in a depression in the surface of the water, but the amount of displacement does not account for the floating. Viscosity, however, may play a part, by increasing the tenacity of the film, the particles of which are so held together, or cohere, that the needle fails to part them. In short, although it is eight times heavier than water, the steel floats.
Another suggestive experiment is that of the grapes in soda-water. Fill a glass two-thirds full with soda-water from an ordinary ‘syphon’ and then drop two or three small grapes into it. The grapes sink to the bottom, but they become restless almost immediately and soon rise to the surface, one after the other. They do not remain there; first one and then the other sinks. This performance will continue for half an hour, the individual grapes rising and falling, not always the whole way, but maintaining a condition of intermittent activity. They become quiet only when bubbles cease to be generated at the bottom, that is, when the carbonic-acid gas has been driven out of the water by the relief of pressure. By watching, it is seen that the bubbles attach themselves to the grapes and buoy them to the surface, where the bubbles break. Sometimes a couple of grapes will collide and cause the adhering bubbles to become detached, so that one or both of the grapes sink. In the end the bubbles become too few to buoy the grapes, so that the latter rise only part of the way; finally, they lie motionless at the bottom. During the early and active part of the performance, the grape will strike the surface of the water and rebound from it as if it were a membrane.
The buoyancy of oil is the physical fact most associated with the first development of flotation, although it is subordinated in the latter phases of the process. Oil has a specific gravity less than that of water, and therefore rises to the surface when mixed with water. The lighter oils range in specific gravity from 0.8 to 0.95, as against the 1.0 of water, so that the margin for buoying particles heavier than water is small. For instance, to make a mixture of zinc sulphide and oil as light as water, it would be necessary, even with the lighter oils, to use from 3 to 15 times as much oil by weight as the blende. This suggests that flotation, even as conducted on the lines of the older patented processes, cannot be due entirely to the buoyancy of oil.
The selective adhesion of oil for particles having a metallic lustre is a decisive factor in the process. It has been said that this adhesiveness is characteristic of sulphides; but it is exhibited by tellurides and by graphite also. Similarly, it has been imputed to ‘mineral’ and to ‘metallic’ particles, but both terms would include substances outside the range of this phenomenon. Apparently it is the metallic lustre that is the decisive factor, for this would include the minerals especially amenable, such as molybdenite, graphite, the tellurides, and the bright sulphides. The effect of this marked preference of oil for lustrous metallic surfaces is intensified by the fact that gases (such as air) have a similar adhesiveness for oil, so that, if present in water, they will join in preventing the wetting of the metallic surfaces. It is an equally important fact that quartz and other gangue-minerals, having a ‘non-metallic’ as against a ‘metallic’ lustre, exhibit the opposite preference: they are feebly adhesive to films of oil, and therefore to those of gas, while they are strongly adhesive to water, that is, they are easily wetted. The reason for this difference is not known; it may belong to the, as yet, mysterious realm of electro-statics, but it is a fact that the curve of contact, or wall-angle, between metallic particles and water is convex while that between earthy particles is concave.
“Whatever the reason for this difference, it can be accentuated by acidification of the water. “Acidified water has a greater wetting power than neutral water.” For this fact also no satisfactory explanation is forthcoming. In some eases, the acid may be supposed to dissolve any coating of oxide on the metallic surface, rendering it more lustrous, while on the other hand, the acidity of the water may give it a corrosive penetration beneath the surface of the gangue.
The addition of acid in quantity produces another effect, namely, effervescence or the liberation of carbonic-acid gas by the reaction with calcite, rhodochrosite, or other carbonates such as are often present in the ore. This generates bubbles that will buoy the metallic particles, whether oiled or not. But water contains air in solution; hence by heating the water, or by diminishing the pressure, it is possible to release the air in the form of bubbles that attach themselves to the metallic particles, like the bladders used by persons learning to swim. Moreover, by a violent agitation of the mixture of ore, water, and oil (if added) it becomes easy to entrain or entangle a large volume of air, which will rise through the mass in the form of myriad bubbles, constituting a foam or froth of varying strength and persistence.
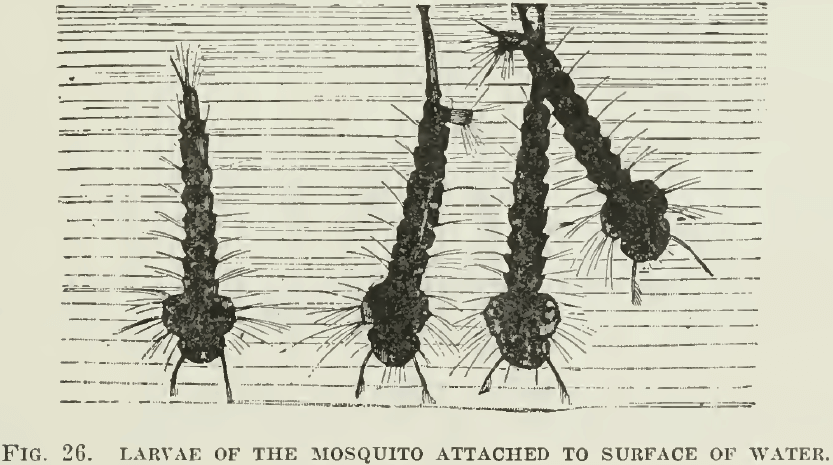
Oil reduces the surface tension of water, that is, between water and air. Pure water has great surface tension, it also has no superficial viscosity; that is why it will not froth. The addition of oil lowers the surface tension and imparts a decided viscosity to the surface of the water. That is why the pouring of oil on troubled waters abates their turbulence. That also explains why the placing of oil in stagnant pools kills the larvae of the mosquito, which then finds it impossible to adhere to the surface by their breathing-tubes. (See Fig. 26.)
Consider the beautiful soap-bubble. The oil of the soap is an impurity that lowers the surface tension of the water; by stretching of the film of the bubble this effect is diminished through the dilution of the impurity, making the film stronger and less prone to collapse. Thus it renders the bubble more persistent. The bubble therefore is another illustration of surface tension, for it is an elastic skin of water enclosing gas, like a balloon. Here also the property of viscosity comes into play, for the addition of oil to the water, more particularly after exposure to the air, as in agitation, gives tenacity to the superficial film. The combination of low tension and high viscosity enables a bubble, rising through the liquid, to envelop itself in the surface film of the liquid, which the tension of the bubble-film is not strong enough to break, so that the bubble endures. The noise made by the bursting of a bubble suggests the fact that it is a receptacle of energy.
The bubble is spherical because the sphere is the shape involving the smallest surface or superficial area. The bubble has an affinity for the lustrous metallic particles and adheres to them, as it also adheres to the smooth sides of a glass. This particle of air, or other gas, is enveloped in an elastic skin of the contaminated viscous liquid in which it has been generated and the metallic particles do not break through that skin for the same reason as the greased needle failed to be drowned in the water.
The addition of soluble oils assists the formation of bubbles in the mass of ore and water. These three constituents of the flotation pulp are mixed intimately so as to form an ‘emulsion,’ such as is typified by mayonnaise. The air present while the emulsion is being made furnishes the gas for the bubbles. In order that they may lift the metallic particles, they must endure long enough to permit complete separation of the metallic particles from the earthy particles, that is, the sorting of the valuable from the non-valuable components of the ore. For the purpose of metallurgical concentration the rate at which the bubbles burst must be slower than that at which they are being formed. An effective froth represents a multiplicity of persistent bubbles. The relative stability of the bubbles depends also upon the kind of oil employed. Pine-oil makes a brittle film; creosote yields an elastic envelope.
By a wonderful correlation of physical forces, the metallic particles become attached to the bubble, made in the metallurgical emulsion, in such a way as to serve as a protective armor, the particles of varying size interlocking on its spherical envelope. The bubbles without mineral are like a balloon with a weak gas-bag, which is likely to burst, while the armored bubbles are like a balloon with a strong gas-bag, which does not burst. Variety of size among the metallic particles favors the construction of the interlocking mineral coat on the bubble, just as materials of various size help to make a dense concrete. Hence slime is no hindrance.
Intimate mixing is required. The more thorough the mixing, the cleaner the separation of the metallic from the earthy particles. This is said to be due to the complete oiling of the metallic particles, but it is a fact that no oil can be discerned on the concentrate when using the 1/3 to ½ pound of oil per ton sufficient in most cases for the purpose of the process. The mixing may be beneficial for reasons other than the oiling of the concentratable parts of the ore; it may cause enough friction to clean the metallic surface; it may promote such a solution of the oil in the water as ensures the formation of the right kind of bubbles for a mineral-carrying froth. Heat, by the injection of steam, increases the miscibility, or ability to be mixed, of the oil, thinning it so that it will extend over a larger surface, as butter is warmed to make it spread over pop-corn. Many common oils, such as ‘red oil’ and other forms of oleic acid, are solid at the ordinary temperature, so that heat sufficient to raise the temperature of the emulsified pulp to about 80° F. is desirable.
To apply this process of concentration, the ore is crushed to the degree of firmness required to separate the metallic minerals from the earthy gangue. This may mean anything from 40 to 200-mesh. The crushed ore is then mixed with water in the ratio, say, of 3:1, although theoretically 2 :1 would make a better emulsion; oil is added, say, in the proportion of ½ lb. per ton of ore; and the mixture is agitated violently in the presence of air, by paddles or beaters, by passage through a centrifugal pump, or by jets of compressed air. Acid is not necessary, as we now know, although it has heretofore been considered requisite. Whether oil is absolutely essential is open to doubt. Agitation of the pulp in the presence of air is the prime factor in producing the desideratum, namely froth. What machines are best adapted to ensure proper agitation is a matter for separate consideration. Aeration of a liquid by agitation in the presence of air and forcing of the air into it so as to form multitudinous small bubbles, producing a froth, is done every day in the domestic operations of the beating of eggs and the whipping of cream.
We have now got our froth. This, as it accumulates on the surface of the emulsified pulp, may be from 2 to 3 inches up to 10 or 15 inches thick. It is so densely coated with the sulphides as to be black, while the gangue that falls to the bottom is so clean as to be white. By skimming, using radial arms or scrapers, or a simple flow, the froth is removed to a secondary receptacle or ‘cell,’ of the spitzkasten or V-shaped type, where it is cleaned by a repetition of the process, making a high-grade concentrate, while the discard goes back for re-treatment. In short, all that is needed is some arrangement for thorough mixing and aeration, by the use of paddles or air under pressure; then the removal of the resulting froth so that the floated mineral will not drop when the bubbles break. When the froth has been collected, it is filtered, yielding a cake containing about 10% moisture, which may be dried before shipment or final treatment for the extraction of the precious metals.
https://archive.org/stream/flotationproces00unkngoog#page/n138/mode/2up
All of the natural phenomena, or appearances, described at the beginning of the previous article, play their part in flotation and each of them has served as the basis for one or other of the many patents that have involved the subject in a maze of vindictive litigation.
SURFACE TENSION
SURFACE TENSION is the idea underlying Hezekiah Bradford’s patent of 1886. In this process the dry powdered ore is caused to meet the surface of a still body of water, so that the metallic particles, which are not wetted, are made to float away, while the gangue particles, which are wetted, sink. This was the first application of flotation without the aid of oil.
In 1904 A. P. S. Macquisten invented a tube apparatus in which surface tension is utilized for concentration. In 1906 the process was applied on a working scale in the Adelaide plant at Golconda, Nevada, where chalcopyrite was separated from a lime-garnet gangue. In 1911 the Federal Mining & Smelting Co. adopted the process for the Morning mill, at Mullan, Idaho, in the separation of blende and galena from a quartz-siderite gangue. At Golconda 96 tubes treated 125 tons per day; at Mullan, 119 tubes treat 150 tons. The iron tube is 6 ft. long by 12 in. diameter. The interior is cast with a helical groove. The tube is revolved at 30 r.p.m. Success appears to depend upon the angle at which the metallic particles are presented to the surface of the water. Subsequently, the water at Golconda was slightly acidified, so that it must have caused an ebullition of carbonic acid gas from the lime in the ore. Thus the bubble phenomena may have come into play. Later, small additions of coal-oil were made, so that another phase of flotation was introduced. In the first instance, however, the Macquisten tube was a real surface-tension process.
In 1905 H. L. Sulman and H. F. K. Picard obtained a British patent for a similar process, but it was a failure. As the floating particles are in the nature of a film, or “in patches one particle thick,” the area of the separating surface has to be large and still. Moreover, some gangue-minerals are floated as readily as the metallic parts’ of the ore.
In 1912 H. E. Wood described his method of concentration, by the surface tension of water alone, in a paper read before the American Institute of Mining Engineers. In common with other metallurgists, he had noticed that dry particles of sulphide minerals are “good swimmers.” In all gravity work, we try to drown them. He had also proved for himself that the oxides are easily wetted. Thereupon he devised a machine in which the dry-crushed ore is fed in a thin stream from a vibrating plate onto a current of water. An impetus is given to the surface by small water-jets. By retarding the current the gangue is made to sink, while the film of sulphides remains on the surface. The elasticity and tenacity of this film is remarkable. The process is being applied on a commercial scale to molybdenite ores by the inventor, Mr. Wood, at Denver. He has also made experimental demonstrations on graphite, tellurides, and other lustrous minerals. At the San Francisco del Oro mill, in Chihuahua, Mexico, 12 of his machines are in use on an ore that has defied other efforts at concentration.
BULK-OIL
BULK-OIL flotation was invented by Robinson & Crowder in 1894 and developed successfully by Francis E. Elmore, whose British patent was obtained in 1898. In the Elmore process the crushed ore is mixed with several times its weight of water. With this pulp a weight of oil equal to, if not exceeding, that of the ore, is mixed gently, so as not to break or emulsify the oil. The oiled mass is run into a spitzkasten, where the oil rises to the surface, buoying the metallic particles, while the gangue and water are removed at the bottom. While oil is described as the prime agent, it is probable that air, entrained by agitation, increased the buoyancy of the concentrate.
OIL AND AIR
Coming to processes using a combination of oil and air, we have the Everson patent of 1885. Carrie J. Everson was washing some sacks in which concentrate had been shipped to her brother’s assay-office at Denver when she noticed that the sulphide particles floated on the water. It is said that the sacks had become greasy, but it is quite likely that she used soap, in which case the greasiness is not required as an explanation. In her process the maximum addition of oil, namely, 18%, is less than one-sixteenth of the quantity required for bulk flotation. As to air, that she obtained by the agitation of the pulp by means of two fans radiating from a hollow revolving tube. The result—according to a description written in 1890, not in the light of prejudiced observation today—was the formation of a “thick scum of sulphides” that “rose to the surface and was skimmed off, leaving the hitherto black ore as white as snow. ”
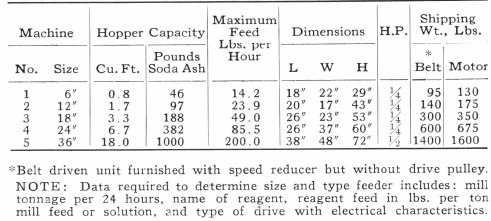
The original bulk-oil process of Elmore had numerous applications, some of which were fairly successful, but in 1904 it was displaced by the Elmore vacuum process, in which flotation by bulk- oil was subordinated to the buoyant effect of air-bubbles generated from the oiled mixture while under a vacuum, and by heating. Under normal conditions water holds in solution an amount of air equal to 2.2% of its volume. This is liberated under a vacuum, but neither the amount of air released (especially at high altitudes) nor the quantity of oil used suffices to explain the degree of flotation achieved, as measured in weight of concentrate. The presence or the addition of limestone or other carbonates, with the use of acid, suggests the aid of bubbles of gas other than air. The proportion of oil in this process has been decreased gradually from 10 lb. per ton of ore to as little as 2.7 lb. per ton. As the mixing involves violent agitation, it seems inevitable that entrained air plays a part.
To the ‘oil and air’ process we must add that of Edmund B. Kirby, for which patent was applied in December 1903 and granted in January 1906. Kirby experimented on ore from Rossland, British Columbia. He used a large proportion (“one-fourth to three-fourths as much, by weight, as ore”) of oil; he added acid; he employed heat; he “thoroughly agitated”; he “injected air into the mass”; and he obtained “a floating scum of hydrocarbon liquid, air, bubbles, and concentrates.” In the light of later events it is claimed that he must have made a ‘froth,’ because the oil was insufficient to cause bulk flotation and the agitation sufficed to entrain enough air to produce a froth. To this the patentees of the so-called ‘agitation- froth’ process reply that his “scum” was not a “froth” in their sense of the term. That he produced froth seems highly probable; but to say that ‘scum’ and ‘froth’ are the same thing, is, in my opinion, not correct.
BUBBLES
Meanwhile the bubble methods of Charles V. Potter and Guillaume D. Delprat had been patented in 1902. In these processes gas was chemically generated with a view to promoting the flotation of metallic particles in Broken Hill ore. This Australian ore contains calcite, which by the addition of acid, emits bubbles of gas that adhere to the sulphides. Potter used acid, agitation, and heat, while Delprat employed a hot solution of salt-cake or acid sodium sulphate and sulphuric acid. Both processes were successful on a large scale, particularly Delprat’s, which is still in use at the Broken Hill Proprietary mine. Neither used any oil. The bubbles attach themselves to the sulphide (blende and galena) particles and carry them to the surface, whence they flow with the liquor into a compartment where, the bubbles breaking, the metallic freight is dropped, and collected as a mixed concentrate. T. J. Hoover says that “the result of the manipulation to which the material is subjected is the formation of a dense froth of bubbles and mineral”; but this was published in 1912, and must be read in the light of events long subsequent to the claims made by either Potter or Delprat. In order to explain the making of froth without oil, he suggests the presence in the ore of such substances as “yield gummy organic compounds that selectively adhere to the ore.” This is an important suggestion. Be that as it may, the Potter and Delprat methods demonstrate that flotation is practicable by the aid of bubbles without the addition of oil.
In the Froment process, patented in Great Britain and Italy in June 1902, the bubble idea is dominant, for, while Alcide Froment used oil, he employed it to attract the bubbles of gas generated by the reaction between acid and calcitc, adding the latter if suitable carbonates were lacking in the ore. He emphasized the fact that not only have the lustrous metallic particles an affinity for films of oil, but the oil itself attracts bubbles of gas. both air and carbon dioxide. He recommends much less oil than had hitherto been used, namely, a “thin layer of oil,” which has been interpreted, according to the exigencies of litigation, to mean anything from less than 1% up to 14%, according as the Froment patent was being upheld or attacked. In Froment’s later instructions to the purchasers (Minerals Separation, Ltd.) of his patent he mentioned the quantity as from 1 to 3£%. For a 5% sulphide ore, the oil would weigh 20 lb. per ton. This question of the quantity of oil required by Froment has been much discussed, but the dominant idea in his mind appears to have been the affinity of oiled particles, necessarily sulphides or lustrous metallic particles, for bubbles of gas. These he obtained by agitation (air-bubbles) and by adding both acid and calcite (bubbles of carbonic acid gas) to the pulp. As to what “a thin layer of oil” may mean, I do not know what Froment intended by the expression, but the scientific meaning of the phrase is indicated by the fact that oil when dropped on the surface of water will spread out in a film one molecule thick.
The agitation of the ingredients specified by Froment will produce a froth; therefore, to the detached onlooker, it is difficult to distinguish the essentials of his process from those claimed in the basic patent of Minerals Separation. Mr. Sulman called the Froment froth a “tender and evanescent assemblage of bubbles of carbon dioxide carrying mineral,” but if it carried mineral I do not see that his refusal to call it ‘froth’ is of any great consequence to those of us who are not interested in the litigation.
COAGULATION
Here we come to what is apparently a break in the sequence of inventivcness, for, beginning with November 1902, Arthur E. Cattermole obtained a succession of patents for a process in which the idea of oil-selection is used to sink the metallic particles of an ore, not to float them. To an acidified pulp he added from 4 to 6% “of the weight of metalliferous matter present,” not of the ore as a whole; therefore, with a 12% zinc ore this would mean 0.48 to 0.72%, say 10 to 15 lb. oil per ton of ore; and with a 2% copper ore, it would mean only 1^ to 24 lb. of oil. But this oil “is brought into the condition of an emulsion in water containing a small percentage of soap or other emulsifying agent.” These are the words of his most important patent, U. S. No. 777,273, dated December 13, 1904, but in his first patent, British No. 26,295, of November 28, 1902, he gives the proportion of soap as 2%. When this mixture of ore, acidulated water, and soapy oil is agitated violently the metallic particles are agglomerated into flocculent masses that sink, the separation from the gangue being then effected by an up-current of water. To facilitate the separation, the mixing was conducted in two stages, of which the second is said to have been “a rolling form of agitation.” Cattermole called his agglomerate a ‘granule’; Froment called it a ‘ spherule. ’
FROTH
The Minerals Separation company was organized in 1903 to acquire the Cattermole invention and thereafter his patents became part of the property of that company. The first and only plant to use the Cattermole process was erected on the Central mine at Broken Hill. where it was soon displaced by the so-called agitation-froth process of Sulman, Picard, and Ballot. These gentlemen have testified that they made their discovery by experimenting with the Cattermole process, applying scientific methods of research, based on the fact that sometimes “loose flocculent masses of partially granulated sulphides” would rise, instead of sinking. Finally, they decided that this was due to insufficient oil. The actual experiments were made by Arthur H. Higgins, who, by diminishing the amount of oil to 0.62% on the ore, caused so many of the metallic particles to rise that a high recovery was obtained by flotation. H. L. Sulman says that by reducing the amount of oil the granulation was stopped and “co-incidentally a mineral froth began to take its place.” This was in March 1905. Whereupon the British patent of Minerals Separation No. 7803, of April 12, 1905, was taken out by H. L. Sulman, H. F. K. Picard, and John Ballot, and subsequently they obtained the U. S. patent No. 835,120 of May 29, 1905, issued on November 6, 1906. 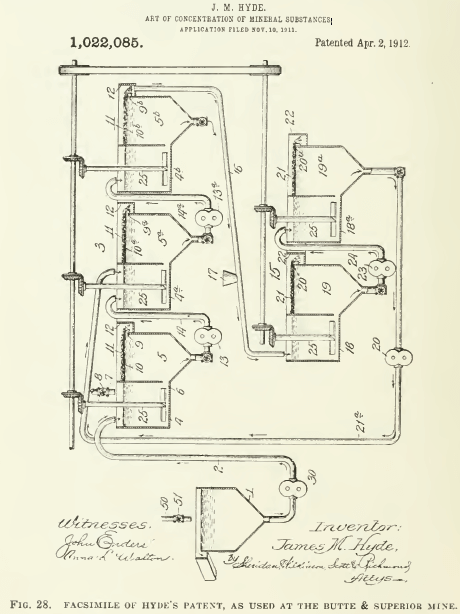 In this patent reference is made to the Cattermole patent and it is claimed that the ‘granulation’ characterizing his method is stopped by reducing the amount of oil to “a fraction of 1% on the ore” and that by vigorous agitation the oil-coated partieles are caused “to rise to the surface of the pulp in the form of a froth or scum.” The use of ‘scum’ here is unfortunate for Minerals Separation, for it tends to identify the ‘froth’ made by this process with the ‘scum’ made by most of their predecessors in the art. In this patent acidulated water, warming of the mixture, oleic acid from 0.025 to 0.5% on the ore, oleie-soap solution, the formation of the froth, and the separating of the froth from the remainder of the solution are specified. Since this patent was issued the process has been applied successfully, and on a large scale, in many parts of the world, notably Broken Hill, Great Cobar, Great Fitzroy, Chillagoe, and Wallaroo, all in Australia; also the Braden copper mine in Chile; and more recently at the Inspiration, Anaconda, and other important mines in this eountry. It is proper to add that a froth-flotation process is used successfully at the Butte & Superior, Miami, and other mines, but the users deny that it is a method to which the Minerals Separation company has proprietary rights. The difference of opinion is yet to be settled by the Courts.
In this patent reference is made to the Cattermole patent and it is claimed that the ‘granulation’ characterizing his method is stopped by reducing the amount of oil to “a fraction of 1% on the ore” and that by vigorous agitation the oil-coated partieles are caused “to rise to the surface of the pulp in the form of a froth or scum.” The use of ‘scum’ here is unfortunate for Minerals Separation, for it tends to identify the ‘froth’ made by this process with the ‘scum’ made by most of their predecessors in the art. In this patent acidulated water, warming of the mixture, oleic acid from 0.025 to 0.5% on the ore, oleie-soap solution, the formation of the froth, and the separating of the froth from the remainder of the solution are specified. Since this patent was issued the process has been applied successfully, and on a large scale, in many parts of the world, notably Broken Hill, Great Cobar, Great Fitzroy, Chillagoe, and Wallaroo, all in Australia; also the Braden copper mine in Chile; and more recently at the Inspiration, Anaconda, and other important mines in this eountry. It is proper to add that a froth-flotation process is used successfully at the Butte & Superior, Miami, and other mines, but the users deny that it is a method to which the Minerals Separation company has proprietary rights. The difference of opinion is yet to be settled by the Courts.
In the foregoing review, I have omitted reference to a number of flotation patents, some of them interesting, because the multiplication and repetition of detail would be only confusing. It will he noted that the amount of oil per ton of ore has decreased from over a ton to less than half a pound. From an insistence upon the use of acid in all the patents, even to the last quoted in the above summary, we come to the recent fact of flotation in alkaline solutions. Indeed, in the case of the Mexican mill we are told that the deleterious effeets of soluble sulphates was overcome by an excess of oil. How much of the oil used in the prior art was due to excess of acid, it remains to be stated by an independent investigator. Much of the early work with flotation was done on Broken Hill ore, which contains a notable proportion of carbonates, hence the addition of acid proved a help, by generating gas, not only in the Potter and Delprat processes, but in others also, namely, those using oil. One ingredient, however, has gained progressively in importance: air. Other gases have had their day, some generated chemically, others electrolyticallv, but in the latest phase of the process the prime agent is air. Indeed, the good results ensuing from the lessened proportion of oil may he due to the fact that the less the oil the greater the intensity of agitation required to spread it throughout the pulp. 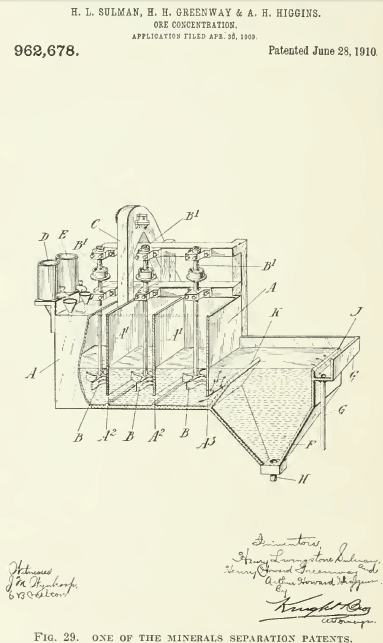 The vigorous agitation, so often emphasized, may have been like the shot that was aimed at the crow and killed the pigeon, for it must have done more than mix the ingredients: it resulted in entraining and atomizing a large volume of air. The later history of flotation suggests that a day may come when the oil, like the acid, will be found non essential, and in its place will be added the ingredient that supplies the substance required for making bubbles. To make bubbles the surface tension of the water in the flotation-cell must be decreased by a contaminant and at the same time the viscosity of the liquid must be strengthened. Oil is not the only substance that can perform these functions. Some alkaline compound may be found that will do the trick. In the Cattermole, Sulman & Picard patent (U. S. 777,274) a fatty acid is produced in situ. In another patent, by Sulman. Greenway & Higgins (IT. S. 962,678) a claim is made for “an organic compound contained in solution in the acidified water” as a soluble frothing agent. In U. S. 1,055,495, Schick claims the use of carbon tetra-chloride to promote ‘levigation,’ or flotation. In U. S. 770,659, Scammell employs sulphur dioxide as a means for increasing the viscosity, and in U. S. 744,322, Foote uses slaked lime. Among other nostrums, alcohol, phenol, camphor, amyl acetate, benzoic and lactic acids, and calcium chloride have been suggested in various patents. In some cases, possibly, an ingredient of the ore itself may suffice. Meanwhile the element of time essential to a good formation of froth suggests that the delay is useful in increasing the viscosity. Mere speed of agitation and aeration does not seem to suffice. But sub-division of the air helps. This reminds us that T. J. Hoover and Minerals Separation took out a patent, in Great Britain in 1910, for the introduction into the oiled pulp of air and other gases through a permeable medium, but it was not deemed worth while to obtain a patent in the United States. Knowing nothing about this, J. M. Callow hit upon the same idea and designed the porous bottom now in use at many flotation plants. Cattermole used an ordinary cone or Gabbett mixer fitted with baffles. Froment employed a mixer of the egg-beater type. Sulman & Picard in one of their patents (U. S. 793,808) suggest an agitator made of a coil of perforated gas-pipe, through which compressed air and oil are fed. Centrifugal pumps, Pachuca agitators, air-jets, and pans with mechanical stirrers have been adopted by various inventors. Other devices for causing agitation and promoting aeration of the pulp have been, and are being, introduced.
The vigorous agitation, so often emphasized, may have been like the shot that was aimed at the crow and killed the pigeon, for it must have done more than mix the ingredients: it resulted in entraining and atomizing a large volume of air. The later history of flotation suggests that a day may come when the oil, like the acid, will be found non essential, and in its place will be added the ingredient that supplies the substance required for making bubbles. To make bubbles the surface tension of the water in the flotation-cell must be decreased by a contaminant and at the same time the viscosity of the liquid must be strengthened. Oil is not the only substance that can perform these functions. Some alkaline compound may be found that will do the trick. In the Cattermole, Sulman & Picard patent (U. S. 777,274) a fatty acid is produced in situ. In another patent, by Sulman. Greenway & Higgins (IT. S. 962,678) a claim is made for “an organic compound contained in solution in the acidified water” as a soluble frothing agent. In U. S. 1,055,495, Schick claims the use of carbon tetra-chloride to promote ‘levigation,’ or flotation. In U. S. 770,659, Scammell employs sulphur dioxide as a means for increasing the viscosity, and in U. S. 744,322, Foote uses slaked lime. Among other nostrums, alcohol, phenol, camphor, amyl acetate, benzoic and lactic acids, and calcium chloride have been suggested in various patents. In some cases, possibly, an ingredient of the ore itself may suffice. Meanwhile the element of time essential to a good formation of froth suggests that the delay is useful in increasing the viscosity. Mere speed of agitation and aeration does not seem to suffice. But sub-division of the air helps. This reminds us that T. J. Hoover and Minerals Separation took out a patent, in Great Britain in 1910, for the introduction into the oiled pulp of air and other gases through a permeable medium, but it was not deemed worth while to obtain a patent in the United States. Knowing nothing about this, J. M. Callow hit upon the same idea and designed the porous bottom now in use at many flotation plants. Cattermole used an ordinary cone or Gabbett mixer fitted with baffles. Froment employed a mixer of the egg-beater type. Sulman & Picard in one of their patents (U. S. 793,808) suggest an agitator made of a coil of perforated gas-pipe, through which compressed air and oil are fed. Centrifugal pumps, Pachuca agitators, air-jets, and pans with mechanical stirrers have been adopted by various inventors. Other devices for causing agitation and promoting aeration of the pulp have been, and are being, introduced.
