Gold Monochloride or Aurous Gold Chloride “AuCl” is a salt is prepared by heating the trichloride to 185° in air for twelve hours. It is non-volatile and unaltered at ordinary temperatures and pressure by dry air, even when exposed to light, but begins to decompose at temperatures above 160°, and the decomposition is complete if it is heated at 175° to 180° for six days, or at 250° for one hour. Its density is 7.4. Water converts aurous chloride into a mixture of gold and gold trichloride. It is a citron-yellow amorphous powder.
Auro Aurichloride
Au2Cl4 is a dark red compound having this composition is said, by Thomsen, to be obtained by heating finely-divided gold in a current of chlorine to 140° to 170°. According to Kruss and Lindet it is merely a mixture of AuCl and AuCl3. It yields gold and AuCl3 if brought in contact with water, ether, or alcohol.
Auric Chloride or Gold Trichloride
How Gold Trichloride AuCl3 is prepared: Trichloride of gold can be prepared, according to Debray, by heating finely-divided gold in a current of pure dry chlorine in a glass tube at a temperature of 300°. The chloride formed sublimes at this temperature and is deposited in the cooler part of the tube in fine red prisms and needles crystallising in the triclinic system. Kruss tried to repeat Debray’s experiments without confirming his results. He found that AuCl3 was formed at 140° to 150°, and was converted to AuCl at about 180° in spite of the presence of an excess of chlorine. At 220° the AuCl was completely decomposed, leaving metallic gold, while up to this temperature a little AuCl3 continued to be sublimed, but towards 300° all action ceased, and the gold remained unattacked by the chlorine at all higher temperatures.
The exact point at which gold ceases to be attacked by chlorine and the rate of volatilisation of the chloride are of great importance in connection with the loss of gold on roasting auriferous materials with salt. The matter has, therefore, been investigated by the author with the following results:—Gold unites with chlorine if placed in the gas at atmospheric pressure at all temperatures up to a white heat, but the action is slight at a red heat. The absorption of chlorine by gold with the formation of chlorides at first increases in rapidity as the temperature rises, and reaches its maximum at about 225°. The fact that gold is attacked by chlorine, and that the chloride is subsequently volatilised at all temperatures between 180° and 1,100°, was proved by means of Deville’s hot and cold tubes, which enable part of the sublimed chloride to be collected. The rate of volatilisation at various temperatures is as follows:
For purposes of comparison, it may be added that when gold is heated in air or coal gas, no gold is volatilised below 1,050°, and only about 0.02 per cent, in 30 minutes at 1,100°, or about one-hundredth part of that volatilised in chlorine at the same temperature.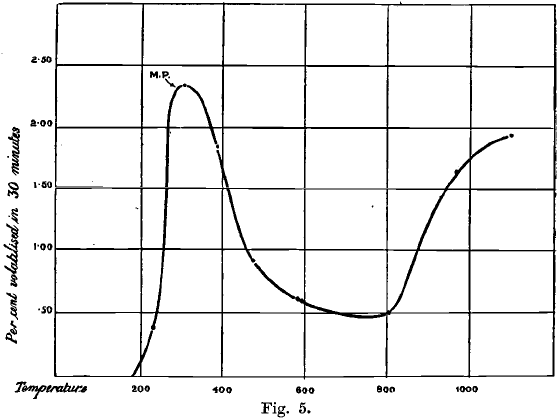
The amounts volatilised vary according to two different factors:
- The vapour pressure of gold trichloride, AuCl3, which of course increases continuously as the temperature rises; and
- the pressure of dissociation of the trichloride, which also rises continuously with the temperature, but not at the same rate as the vapour pressure.

The rise of vapour pressure tends to raise, and that of the pressure of dissociation to reduce, the amount of gold volatilised as chloride. The vapour pressure increases more rapidly than the pressure of dissociation at temperatures below 300°, and also above 900°, but less rapidly at intermediate temperatures. Hence the curve (Fig. 5) showing the variation of volatilisation with temperature is irregular, passing through a maximum near 300°, and a minimum at a point somewhere below the melting point of gold. The first named change in the direction of the curve probably occurs at the melting point of the chloride—namely, 288°. The second change is perhaps caused by the change of sign of the heat of formation of the trichloride AuCl3 ; when this becomes negative, the pressure of dissociation of the compound would decrease, in accordance with the law of van’t Hoff and Le Chatelier. However this may be, it is certain that when gold is heated in chlorine at atmospheric pressure, trichloride of gold is formed and volatilised at all temperatures above 180°, up to, and probably far beyond, 1,100°.
The usual method adopted for the preparation of auric chloride is to dissolve gold in aqua regia, and then to evaporate the liquid to dryness, keeping the temperature as low as possible. A brownish-red mass is thus formed, consisting of AuCl3 mixed with more or less of the protochloride and of hydrochloric acid. On taking up with water the protochloride is decomposed into gold and trichloride, but the hydrochloric acid can only be eliminated by shaking with ether, which withdraws the tri¬chloride from its solution in water. If an attempt is made to drive off the hydrochloric acid by heat, a partial decomposition of the trichloride results.
Auric chloride exists both in the anhydrous state and in combination with two equivalents of water, AuCl3 . 2H2O, when it occurs in orange-red crystals. The anhydrous salt is of a brilliant-red colour, crystallising in needles belonging to the triclinic system, and melting at 288° under a pressure of two atmospheres of chlorine. It can be prepared by drying the hydrated salt at 150°. The anhydrous and hydrated salts are both hygroscopic, and dissolve readily in water with elevation of temperature; they are also soluble in alcohol and ether, and in some acid chlorides, such as AsCl3, SbCl5, SnCl2, SiCl4, &c.
Auric chloride is readily decomposed by heat. Lowe states that 4 grammes of the trichloride, when heated in a porcelain basin on a boiling water bath, can be completely transformed into the monochloride, although not until after the lapse of several days. On the other hand, as has already been mentioned, Kruss states that the decomposition of auric chloride, in an atmosphere of chlorine, begins at 180°. According to the experiments of the author, auric chloride is observed to suffer slow decomposition at as low a temperature as 165° in an atmosphere consisting of chlorine, about 1.6 per cent, being converted into monochloride in four hours at this temperature; the decomposition is about five times more rapid at 190°. The decomposition in air can be readily observed at 100°, although it does not seem to be so rapid as was indicated by Lowe. In seven days only 6.6 per cent, of the trichloride was decomposed, the initial rate of decomposition being 0.041 per cent, per hour. At 165°, however, the initial rate of decomposition appeared to be 3.2 per cent, per hour, and the conversion into monochloride was complete in four or five days at 160° and in ten hours at 190°.
Solutions of gold chloride have also been shown to be decomposed by heat, a solution of one part of the chloride in 15,000 parts of pure water yielding a precipitate of gold on heating for some hours. The solution was found to contain traces of hydrogen peroxide, the reaction being expressed by the equations:
AuCl3 + 2H2O = AuCl + 2HCl + H2O2.
3AuCl = AuCl3 + 2Au.
A similar change, yielding hydrogen peroxide, was found by Sonstadt to take place when solutions containing 0.04 per cent, and also 0.007 per cent, of gold chloride were exposed to bright sunshine for several days. The presence of free hydrochloric acid prevents this decomposition. Weak voltaic currents precipitate metallic gold from the solution of the trichloride upon the negative pole. The solution of trichloride of gold is also decomposed by many reducing agents, such as most organic substances, metals, and protosalts; heating the solution in every case hastens the decomposition. The reduction by organic matter is assisted by the action of light, which is especially efficacious in the presence of starchy and saccharine compounds, or of charcoal or ether. In direct sunlight the last reagent deposits a bright mirror of metallic gold, but under ordinary conditions red colloidal gold (Faraday’s gold) is set free. Alkalies also quicken the action of organic matter, and it may be said that all organic compounds reduce gold chloride on boiling in the presence of potash or soda, while Muller states that a mixture of glycerine and soda lye is one of the best precipitants for gold chloride, separating the metal completely in highly dilute solutions. According to Kruss, if potash and soda are quite free from organic matter, they have no action on solutions of auric chloride, whether cold or hot. If a small quantity of organic matter is present, sub-oxide of gold is precipitated; if larger quantities are present, both metallic gold and sub-oxide are precipitated in the cold, but gold alone at boiling point. Alkaline carbonates are without action on cold solutions, but if they are hot, then half the gold is precipitated as hydrate, while the other half remains in solution in the form of a double chloride of gold and the alkali. The precipitation by means of charcoal is of especial importance in view of its adoption in practice.
https://www.youtube.com/watch?v=-rbz3uLZvPc
It has been stated that a current of hydrogen gas will precipitate gold completely, especially on boiling the solution, but Kruss has proved that, if the hydrogen is quite pure, it has no effect either on cold or hot solutions. Sulphur, selenium, phosphorus and arsenic all precipitate gold on boiling the solution of the trichloride. Many metals reduce chloride of gold, the action being, of course, most rapid in the case of the most highly electropositive metals, such as zinc and iron. Lead sometimes gives fine dendritic plates of gold. Sulphuretted hydrogen precipitates sulphide of gold from both neutral and acid solutions, all traces of gold being readily removed from a solution by this reagent, whilst phosphoretted, arseniuretted, and antimoniuretted hydrogen all precipitate metallic gold. The lower oxides of nitrogen, nitrous acid and many other “ ous ” acids and oxides effect the same decomposition. Sulphur dioxide is a convenient reagent, and is often used in the laboratory, being almost equally efficacious in cold and hot solutions. The reaction is:
2AuCl3 + 3SO2 + 6H2O = 2Au + 6HCl + 3H2SO4.
Various protosalts also reduce trichloride of gold. Ferrous sulphate is often used to detect the presence of gold in solution as chloride; this reagent gives the solution a pale blue colour by transmitted light, and brown by reflected light, owing to the formation of finely-divided precipitated gold. The reaction is represented by the following equation:
2AuCl3 + 6FeSO4 = 2Au + 2Fe2(SO4)3 + Fe2Cl6
To test a dilute solution for gold, a test tube filled with the liquid is held in the hand side by side with a test tube filled with distilled water and a few drops of a clear solution of ferrous sulphate are added to each. On looking down through the length of the test tubes from above, with a white surface as background, any slight changes of colour may be detected by comparison, and the liquid may also be compared with the original solution in a test tube. In this way, by a little practice, the presence of gold in the proportion of only (1 dwt. per ton of water), or even less can be detected. The method is often used in the chlorination process, but it is better to use protochloride of tin, SnCl2. This substance gives a brown precipitate of variable composition in concentrated solutions, but if mixed with the tetrachloride, SnCl4, it gives a precipitate of purple of Cassius. The reaction is very sensitive, and by its means a violet coloration by transmitted light can be obtained in a solution containing 1 part of gold in 500,000 parts of water, while by special means the presence of 1 part of gold in 100,000,000 parts of water can be detected, as described below. The liquid supposed to contain gold is raised to boiling, and poured suddenly into a large beaker containing 5 to 10 c.c. of saturated solution of stannous chloride, and the liquids agitated so as to effect complete mixture. A yellowish- white precipitate of tin hydrate forms, which settles rapidly, and can be readily separated from the bulk of the liquid by decantation. If the solution originally contained at least 1 part of gold in 5,000,000 of water (3½ grs. per ton), the precipitate is coloured purplish-red or blackish-purple, according to the nature of the solution, and the condition of the precipitant. The colour can be seen without comparing it with other precipitates. If less gold than this is present it is better to compare the precipitate with one obtained by the use of boiling distilled water, and to increase the quantity of liquid used while adhering to the same amount of stannous chloride. In this way the presence of 1 part of gold in 100,000,000 parts of water (1 grain of gold in 6 tons of water) can be detected, the amount of liquid required in this case being about 3 litres. The gold is concentrated in the precipitate in which a distinct colour is caused by less than 0.05 per cent, of the precious metal.
Chloroauric Acid
Chlor-auric Acid, HAuCl4.—Gold trichloride in the presence of free hydrochloric acid forms this compound, which crystallises out on evaporation in vacuo in long yellow needles, having the composition HAuCl4 + 4H2O, and since gold chloride unites with many other soluble chlorides to form double chlorides, this hydrochloric acid compound is regarded as an acid. It is more stable than gold trichloride. The chlor-aurates, having the general formula M’AuCl4, or AuCl3. M’Cl, are readily soluble bodies which can be crystallised, and which decompose with about the same readiness as chlor-auric acid. The sodium salt, NaAuCl4. 2H2O, is used for “ toning” in photography.
