Mineralogy & Microscopy
Amenability of an ore to flotation is best tested for by a microscopic examination of the ore followed by a few laboratory flotation tests. As stated in Chapter I, most ores containing minerals of metallic, resinous or adamantine luster associated with minerals of earthy, vitreous, or pearly luster, can be divided by froth flotation in such a way that the floating part will contain the bulk of the mineral or minerals of metallic, resinous or adamantine luster to the practical exclusion of the associated minerals, while the part not floated will consist largely of the associated gangue minerals. For microscopic examination, thin sections of the ore, such as are prepared for petrographie work, or polished sections should be examined through a suitable microscope to determine the method of occurrence and grain size of the valuable mineral. Fragments of the ore crushed to pass a 0.295-mm. screen (or its equivalent) should be examined under a binocular microscope at 20 to 50 diameters magnification and also under the petrographic microscope. The examination of the fragments will give a good idea of the mineralogical character of the ore. Rogers’ “Study of Minerals” (see page 23) is a good guide for this determination. The examination of thin or polished sections will tell whether alteration has taken place at the surface of the minerals that it is desired to float. If microscopic examination shows unaltered sulphides associated with the ordinary rock-forming gangue minerals, it is safe to conclude that the ore is amenable to flotation.
This conclusion can probably be confirmed by a few laboratory flotation tests as follows: Machine—Janney laboratory flotation machine. Ore—500 gm. ground to pass a 0.295-mm. screen. Flotation agents—About 0.3 cc. of oil.* Follow the procedure described. The appearance of the froth through the glass sides of the separating compartment compared with that of the pulp will indicate whether concentration is going forward. A better measure is afforded by a microscopic examination of the froth or by examination of the same by vanning on a plaque or watch glass.
If the concentrate obtained is low-grade, it can probably be improved by increasing the percentage of moisture (lessening the weight of solids charged) or by adding petroleum residuum or low-grade kerosene (stove oil) to the oil mixture, or, except in the case of a carbonate gangue, by the addition of sulphuric acid in the proportion of from 4 to 10 lb. per ton of solids. If recovery is low due to dropping of sulphides from the froth, which will be apparent from examination through the glass side of the separating box during the progress of the test, it can probably be improved by decreasing the proportion of pine oil in the oil mixture or by increasing the ore charge. If the froth is too effervescent decrease in pine oil or addition of petroleum or wood-tar oils will add stability. Finer grinding may also aid, or the addition of sulphuric acid. Reference to the following tabulation of oils and other principal flotation agents classified according to properties will serve as a guide to the oil changes. In any case, if the preliminary microscopic examination has revealed a mineralogical composition of the type amenable to flotation, persistence in the search for proper operating conditions should result in the discovery of a suitable method.
TABLE OF PRINCIPAL FLOTATION AGENTS CLASSIFIED ACCORDING TO
THEIR CHARACTERISTIC TENDENCIES IN FLOTATION
Agents which tend to produce a voluminous froth:
- Essential oils (steam-distilled pine oil, eucalyptus oil).
- Tar acids (phenols, cresols).
- Amyl acetate and amyl alcohol.
Agents which tend to enhance selection of sulphide minerals:
- Petroleum and petroleum derivatives.
- Coal tar and coal-tar oils.
- Wood tar and wood-tar oils and creosotes.
- Nitrogenous bases from tars.
Agents which tend to make stiff froths:
- Wood tar and wood-tar oils.
- Petroleum and petroleum derivatives.
The above list does not pretend to be complete. Neither is it to be understood that the classification is a rigid one. Thus while a considerable volume of froth, and a certain degree of stability of the same together with selection of the sulphide mineral are essential to all successful flotation operations, one oil, as for instance pine oil or coal tar, may serve for all three purposes. In general, however, best results will be obtained by a proper admixture of two or more agents.
Insofar as we know at the present time, and the experimental data are convincing, a flotation oil which is to be depended upon for froth formation must be soluble or possess an appreciable soluble portion, and the dissolved substances must have the power of lowering the surface tension of water when added thereto in small quantities. If the oil is to be depended upon for sulphide selection it must adsorb strongly and rapidly at the surfaces of substances of metallic, resinous and adamantine luster and feebly or not at all at the surfaces of ordinary gangue minerals under the conditions of froth- flotation. The effective properties of the agents which stiffen froths are not so well known. It is probable, however, that they adsorb strongly at bubble surfaces and that when spread out in thin films their viscosity is high.
Tests for a process for treatment of an ore
Testing for process development will always proceed most rapidly if started by a careful microscopic examination which will furnish the following information:
- Mineralogical composition.
- Petrographic structure.
- Degree of alteration of the surface of the mineral to be floated.
- The size to which the ore must be crushed in order to free a considerable part of the mineral to be floated from the associated gangue.
This information being at hand, systematic testing should be started with the following facts in mind:
- In the laboratory the agitation-froth process is easier to control and tests are more quickly run than by the pneumatic process.
- An ore that can be successfully concentrated by flotation in an agitation-froth machine can be successfully concentrated, with certain changes in the accessory details of operation, in a pneumatic machine.
- Before a mill is built the process worked out in the laboratory should be tried out on something approximating a mill scale in a test plant.
- The flotation process operates most easily and with greatest leeway on a pulp containing from 15 to 20 per cent, solids. On the other hand, power consumption, mill equipment and oil consumption are lessened as the percentage of solids in the pulp is increased.
- A change in the oil mixture in an operating mill may be a serious matter, involving considerable laboratory experimental work and costly interference with mill operation. Hence the oil chosen should be one of which a supply at a fair price is reasonably assured, and the oils tried in the testing work should be of this class. The important members of the class are: pine oil, coal tar, coal-tar creosote, wood tar, wood-tar creosote, petroleum residuum, and the low-grade kerosene commonly known as stove oil. All of these substances are available at fair prices and in good supply practically everywhere. Certain other substances may be locally abundant and their use, temporarily at least, may be justifiable on that score, but a suitable flotation agent consisting of one or more of the above mentioned substances should be determined and the best available supply investigated against the time when the supply of the local substance is exhausted.
No general rule can be given as to the kind of oil applicable to a given class of ore. Certain general tendencies are, however, to be observed from a study of the kind of oil used in connection with certain ores at various mills throughout the country. In a majority of the mills treating ores containing chalcocite, coal tar or coal-tar creosote forms a considerable part of the oil mixture, with pine oil usually present in minor quantities. In the mills in which galena or sphalerite or both are recovered, wood tar or wood-tar creosote is used in a considerable number of cases. In the treatment of some such ores, pine oil and crude kerosene are employed. In mills using 20 to 25 lb. of oil per ton of ore, petroleum residues form a large part, i.e. upwards of 80 per cent of the oil mixture, with pine oil or coal-tar creosote, or both, forming the balance of the mixture.
The practice in the addition of flotation agents other than oils is no more subject to generalization with regard to different types of ores than is the question of the kind of oil. The only general statement that seems to be founded in experience is that a small amount of copper sulphate solution, say such an amount as will introduce into the pulp somewhat under 0.1 lb. of copper per ton of solids, seems to improve results with sphalerite ores. Sulphuric acid is usually employed with blende ores. Sodium silicate is sometimes used where preferential separation of galena from sphalerite is practiced. A majority of the tonnage of low-grade chalcocite ores is probably being treated in neutral or slightly alkaline circuit, but the exceptions to this rule are so numerous and the results obtained on such ores in acid circuits are so excellent, that no general rules can be formulated. Acid salt cake is used in place of sulphuric acid in some instances, notably where it is considerably cheaper or where there is a considerable amount of carbonate in the ore and treatment in an acid pulp seems to be necessary. It is not unlikely, however, that in the latter case, further investigation would reveal the fact that an acid pulp was unnecessary and that the use of both salt cake and sulphuric acid could be done away with. The great majority of ores that can be concentrated by flotation will give a good flotation result with a mixture of pine oil and coal-tar creosote, the pine oil forming from 5 to 50 per cent, of the mixture. In laboratory tests in a mechanical machine from one to two pounds per ton of ore of such a mixture is necessary and sufficient.
The procedure for preliminary testing, then, should be as follows:
- Grind the ore, best dry, so that all will pass the screen whose aperture corresponds to the average maximum size of grain of the mineral to be recovered, but in any case so that all will pass a 0.3-mm. screen. The grinding should further be such that at least 50 per cent, of the material will pass a screen whose aperture is one-quarter that of the limiting screen.
- Use an agitation-type machine which will give a peripheral speed of impeller tip of from 1500 to 2500 f.p.m. in the case of a three- or four-inch impeller, slightly less in case of a larger machine. The machine should be one that can be easily and thoroughly cleaned. The Janney laboratory flotation machine rigged with the shaft extended through an overhead bearing (see Fig. 4) driven by a variable speed 1/4-h.p. motor is, in the writer’s opinion, the best agitation-type laboratory machine.
- Use such a quantity of ore as will give about 20 per cent, solids at the beginning of the test. The solids-water ratio will, of course, grow less as the test proceeds and concentrate is removed, since the volume of pulp in the machine must be kept up by the addition of water.
- Follow now the procedure of the tests outlined on page 61, using the pine oil-coal-tar creosote mixture, recording all data and observations on each test and carrying each test through at least to the point where assay samples of the products are on the shelves available for assay. It will be wise for the inexperienced operator to assay samples from many of his early tests although he may not believe that the results warrant the labor and expense. It is told by the engineer who directed the experimental work in the early days of flotation at one of the big mills of the country, that he and his staff watched the operation of a pneumatic cell for several days and never considered the work sufficiently good to warrant an assay, but allowed the concentrate to flow with the tailing to waste. Finally they decided that nothing could be done with the machine and he, merely for the purpose of obtaining a definite figure for report purposes, took a sample of the tailing for assay. The assay report showed the tailing to be considerably better than any that had been made on the agitation-type machines running in the mill. Further work, now with assays to indicate the character of the results, confirmed the first assay and demonstrated the utility of pneumatic treatment for the ore in question. The moral need not be pointed.
When a satisfactory flotation mixture that can be used as a standard has been discovered, as it should be in the course of ten or fifteen tests unless the ore is extremely difficult to float, further laboratory investigation should be carried out along the following lines: treatment in a bubble-column machine; determination of the maximum percentage of solids that will give a good grade of concentrate and a satisfactory recovery; the effect produced by the substitution in whole or in part of cheaper flotation agents; the minimum amount of flotation agent that can be used.
As to the first of these lines of investigation, power and flotation agent consumption on a given ore are generally less in a bubble-column machine than in an agitation-type machine and the operation of the former is both more simple and more flexible. It is the author’s opinion that ten years’ time will see the agitation-froth method of flotation almost completely displaced, except in the smallest mills, by some bubble-column method.
For investigation in the laboratory of the method of treatment of the ore in question by the bubble-column process, the machine shown in Fig. 10 is wholly satisfactory. The charge of ore is best ground with the flotation agent in a laboratory ball mill in the presence of an equal weight of water. The solid charge in a machine of the size shown should be about 1500 gm. Sufficient water should be added to make the pulp in the cell about 20 per cent, solids. The air quantity should be so regulated as to maintain a gentle overflow of froth, adding water as the test proceeds to keep up the bulk of the pulp to such a point that the froth overflow can be maintained without excessive disturbance of the pulp in the cell. Use the flotation agent discovered to be best in the agitation cell. Continue the treatment until it becomes apparent that the recovery being made is not commensurate with the time spent in making it. Continue with tests of this character until a standard of performance for the machine has been set up.
Attempt now a substitution of petroleum for a part of the pine oil in the flotation-agent mixture. The apparent result should be decreased effervescence of the froth and a dirtier concentrate. The decreased effervescence need cause no worri-ment unless an excessive amount of air is necessary to maintain a froth overflow, and the dirtiness of the concentrate can probably be corrected in the inevitable cleaning operation. The object sought in these tests should be a tailing of the same grade as that obtained with the standard mixture. If the diminution of the pine oil decreases frothing too greatly it may be possible to get the frothing effect more cheaply with coal-tar creosote or wood-tar creosote. This investigation should be continued until the mixture containing the greatest possible proportion of the cheaper agents, which will produce a recovery and grade of concentrate approximating that obtained with the more expensive standard, is found.
Investigation should now be directed toward the effect of increasing the percentage of solids in the pulp. Such increase will, as previously stated, result in an increase in the tonnage that can be handled through a given machine in a given time, thus saving installation and power expense. It will also decrease the amount of flotation agent necessary and may make possible coarser grinding. The first apparent effect of an increase in the percentage of solids in the feed will be a dirtier concentrate. This can, however, be easily cleaned up in the more dilute pulp in the “cleaner” machine.
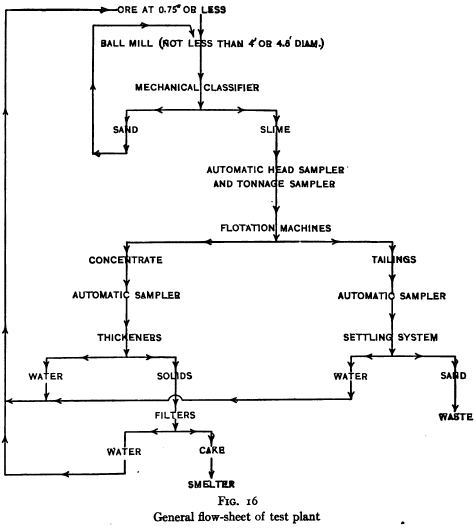
When the laboratory investigation has proceeded thus far the methods developed should be tested out in a test mill, built at the plant, using the water that is to be used in the final plant. A satisfactory test mill should be capable of treating 50 to 100 tons per day and should have a maximum of flexibility, with provision for taking tonnage and assay samples with the least inconvenience and interference with results. While each test mill will vary from every other in its details, the general flow sheet and arrangement of the flotation part of a test mill should be approximately as shown in Fig. 16.
Flotation Testing of Oxidized Ores
By oxidized ores, as the term is commonly used in flotation practice, are meant the oxides, carbonates and silicates of lead, zinc and copper. Tin oxide, cassiterite, also offers a problem, although a different one, in all probability, from the others.
Methods proposed for the treatment of oxidized ores group themselves under three different heads:
- direct flotation,
- those depending upon the formation of a film of a compound of metallic luster on the surface of the valuable oxides and
- those dependent upon the solution of all of the valuable oxides and their subsequent precipitation as metal or sulphide, i.e. as compounds with a metallic luster.
All of the obvious chemical methods of filming and of dissolving and re-precipitating have been tried and patented. Some of the patented methods work with given ores but none are generally successful. No method of procedure that promises successful results can, therefore, be set forth here. For those, however, who are going to embark on the search it may be said that experience points to success along the lines of solution and reprecipitation or of direct flotation, rather than of filming. Copper carbonates can be floated apparently as such from ordinary gangues in the form of a low- grade concentrate in such an amount as to make a real recovery. Also metallic copper and copper sulphide precipitated from solutions of copper salts can be floated. Precipitation is, however, hard to regulate in the presence of the associated pulp and subsequent flotation also presents considerable difficulty.
https://www.911metallurgist.com/flotation-process-development-laboratory
