The oxidation-reduction (dissolution and precipitation) of gold from Au –> Au+ +e- is driven by this equation. Without using a gold periodic table or a oxidation potential chart, you can simply review the chemistry here: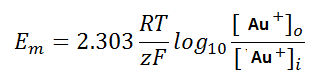
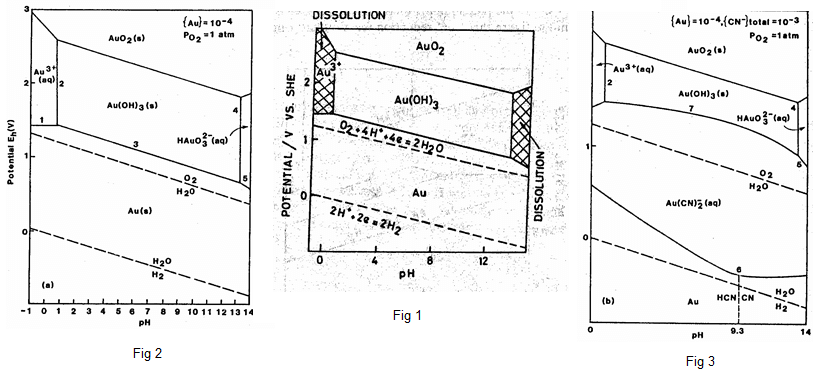
Figure 1 is Au–H2O system: No stability region for gold ions between lines; can’t dissolve Au in aqueous solutions (for now)
Figure 2 shows why we can’t dissolve gold; Figure 3 shows how we can (This is why cyanide is used).
- Vertical line at bottom for H+ + CN– = HCN (g); impacts other lines.
- Curvature of lines; reflects changing activity coefficients
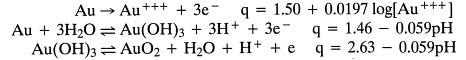
The redox potential of this system VS pH is shown in Figure 1
Learn all about oxidation and reduction:
Witness the magic of gold oxidation:
View the metallurgy gold reduction process:
