The usual purpose of oil testing in flotation work is to determine that the oil in question is similar in physical properties and therefore probably in its behavior in the flotation cell to a given prior shipment, or in order to set specifications for flotation oil purchase. The following tests will give such information.
Color
Color is best tested by comparison in containers of the same size and shape with a sample of the oil with which comparison is to be made. In case no reference sample is available, a general specification as to color, without reference to a color chart, is all that is justifiable.
Limpid point
Take a sample of about 10 cc. in a thick-walled test tube. Cool gradually, at the same time stirring constantly, and lift the oil with a thermometer in such a way as to allow it to flow down the sides of the test tube. Cooling should be continued until crystals can be seen in the oil as it flows down the side of the tube. Note and record temperature at which crystallization starts, and also that at which complete solidification occurs on further cooling. Now warm gradually, stirring constantly as before, until crystals disappear and note temperature of disappearance.
Specific gravity
Place sample of oil in a cylinder 1 to 1 ½ in. diameter and about 6 in. deep. Bring to a temperature of 15° C. Float a hydrometer in the oil, taking care that it does not strike the sides of the cylinder, and read the specific gravity of the sample from the hydrometer scale. Repeat the determination at temperatures of 25 and 60 degrees.
The following method should be followed in the determination of specific gravity by means of a specific gravity bottle. In ordinary work a 25-cc. bottle will be satisfactory. Clean the bottle thoroughly with chromic acid solution, rinse with water and dry. Weigh dry bottle to 1/10 mg. Fill with distilled water at 15° C. Clean and dry the outside of the bottle thoroughly-and again weigh with the same degree of accuracy. Next dry the bottle and fill with oil, bring to the same temperature as that of the water in the previous weighing, clean and dry the outside of the bottle and weigh. The weight of oil divided by the weight of water gives the relative specific gravity of the oil compared with water at that temperature. Repeat at 25° and 60° C.
Viscosity and viscometer
The viscosity of a liquid is the transient resistance offered by the liquid to deformation. The coefficient of viscosity is the ratio of the shearing stress during such deformation to the rate of shear, expressed in proper units, and is a constant for any one fluid under given conditions of temperature and pressure. The “specific viscosity” is the ratio of the viscosity of the fluid under consideration to the viscosity of water at a specified temperature. The latter value is the one which is of interest in testing and classification of flotation oils and the temperatures of principal interest to the flotation operator are those at which the oil is received, at which it is stored, at which it is to be transported from storage to the flotation cell, and that of the flotation pulp in which it is to be used. The apparatus employed in the determination varies according to the viscosity of the oil under investigation. For relatively viscous oils, some form of the Engler viscosimeter is ordinarily employed. For relatively mobile oils, the Ostwald apparatus is used.
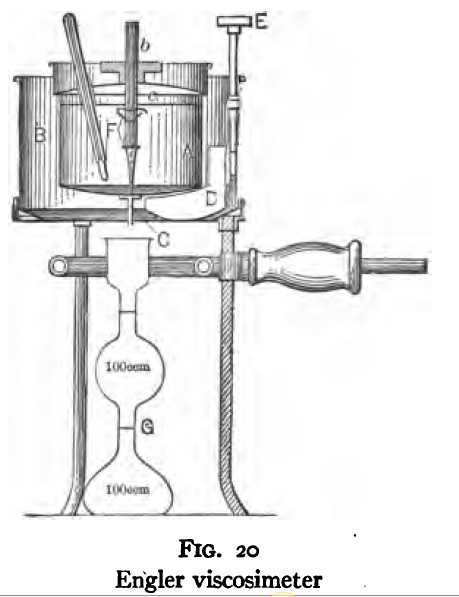
The Engler viscosimeter is illustrated in Fig. 20. It consists essentially of a cup. (A), fitted with a pipe orifice (C), a container (B) with a stirring mechanism (E, D), and a graduated flask (G). The cup (A) is provided with an air insulated cover (c) and a pointed wooden stopper (b).
The following is the procedure for the determination of the specific viscosity of an oil with this apparatus. Take a sample approximately 15 per cent, greater in bulk than required to fill the cup (A) to the top of the gauge points. Strain the sample through a 100-mesh wire screen to remove foreign matter. Clean the cup (A) with solvents such as benzol or alcohol in order to remove any oily substances, taking particular care that the discharge orifice is thoroughly clean and dry. Soft tissue paper or filter paper is best for this cleaning process. Now assemble the apparatus, placing the pointed wooden stopper in the orifice and fill the inner cup to the top of the gauge points with the oil to be tested. Place cover (c) in position and insert thermometer (F). Fill chamber (B) with an oil of higher boiling point, usually a heavy lubricating oil, and insert a thermometer in this liquid. Heat slowly by means of the burner (H), stirring the heating liquid by means of the stirrer (E, D) until the desired temperature is reached. Hold the temperature at this point until the oil in cup (A) has reached the same temperature. Place container (G) in such a position under the orifice that the oil will flow down the side of the container and thus prevent the formation of a froth. Lift the pointed wood stopper and determine with a stop watch the elapsed time in filling the flask to the graduation mark. Repeat until concordant results are obtained. The time required to discharge an equal volume of distilled water under the same conditions of temperature and head on the discharge orifice should now be determined, first cleaning the inner cup thoroughly with oil solvents. The ratio of the time of outflow of the oil to that of the water is taken as the specific viscosity of the oil, referred to water at the same temperature, and is called the “Engler degree.”
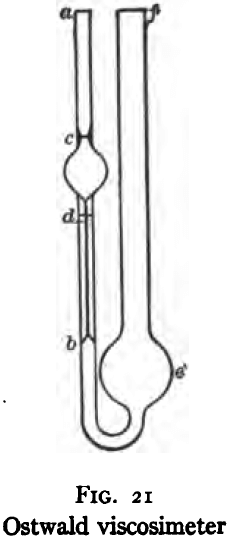
The Ostwald viscosimeter used for mobile liquids is shown in Fig. 21. It consists of a fine capillary tube (db) about 10 cm. long and 0.4 mm. bore, connected at the upper end to a bulb containing a definite volume between the graduation marks (c) and (d). The capillary is connected at the lower end by a U-tube to a large bulb (e), which in turn is connected to a tube of convenient size for filling. Before using, the apparatus should be cleaned first with suitable oil solvents such as benzol, acetone, ether, or alcohol and then with warm chromic acid solution, which latter should be allowed to stand in the apparatus for an hour or more. Finally it should be washed out with water and dried thoroughly by means of a current of air. To make a determination of specific viscosity, introduce into the leg (f) by means of a calibrated pipette, a definite volume of oil sufficient to cause the bulb (e) to stand two-thirds full. Place the viscosimeter in a water bath maintained at a definite constant temperature and allow it to stand therein until the oil and apparatus are of the same temperature as the bath. The time required will range from 30 minutes to 1 hour, according to the difference in temperature between the bath and the surrounding atmosphere. Next place a rubber tube at (a) and suck oil into this limb to some point above the mark (c). Remove the tube and allow the oil to flow down through the capillary (db) by gravity, taking the elapsed time by stop watch for the meniscus to pass from graduation (c) to graduation (d). This operation should be repeated until concordant results are obtained. A variation in elapsed time of 0.1 to 0.5 per cent, is allowable. Repeat the above described procedure with water at the same temperature, using the same volume of water as of oil. The ratio of time of flow of oil multiplied by the specific gravity of the oil, to the time of flow of the water multiplied by the specific gravity of the water gives the specific viscosity of the oil compared with water at the given temperature.
Tar acid determination
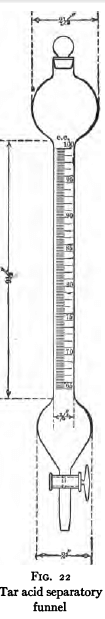
This should be made on all tars and tar derivatives. The method of procedure is as follows: Place 100 cc. of oil in the separatory funnel shown in Fig. 22. Add from 30 to 50 cc. of sodium hydroxide solution (sp. gr. 1.1) and mix by turning the funnel end for end several times. Allow the liquids to stratify roughly, and draw off the lower layer. Repeat this operation with new 30- to 50-cc. portions of sodium hydroxide solution until the aqueous layer, on stratifying, shows no further coloration. Read the volume of residual oil in the separatory funnel. The decrease in volume gives the volumetric percentage of tar acids extracted. Now wash the combined alkaline extracts with C. P. benzol in the separatory funnel, allow the mixture to stratify, and draw off the aqueous layer. Heat this portion on a steam bath until the odor of benzol has disappeared. Cool, place in the separatory funnel and liberate the tar acids with 20 per cent, sulphuric acid solution. Allow to stratify and read off the volume of tar acids liberated.
With some oils preliminary distillation is necessary in order to later obtain separation of the alkaline solution from the oil. In such cases distill 100 cc. of the oil, continuing the distillation until at least 95 per cent by volume of the oil has distilled over or until a temperature of 360° C. has been reached. Read the volume of the distilled oil and determine the tar acids on this portion by the procedure above outlined. Calculate on basis of original bulk.
Sulphonation
This is used for the purpose of determining unsaturated and aromatic constituents in an oil. Its chief use is for determining adulterants, such as mineral oils, in pine and tar oils. The test is carried out by placing 2 cc. (accurately measured) of the oil in a Babcock cream-testing bottle and treating the oil with 37-normal sulphuric acid in small quantities, shaking the oil and acid mixture thoroughly after each addition. When no more heat is developed on the addition of acid and when about 40 cc. have been added, heat for one hour at a temperature of 98° to 100° C. The bottle may then be filled to the upper graduation with sulphuric acid (sp. gr. 1.84). The mixture should now be centrifuged for five minutes and the volume of residual oil read from the graduations. The volume of residual oil divided by the original volume represents the percentage of unsulphonated material present in the original oil. This volume should be not over 3 or 4 per cent. for a good steam-distilled pine oil. For tars, the volume will vary considerably.
Distillation test
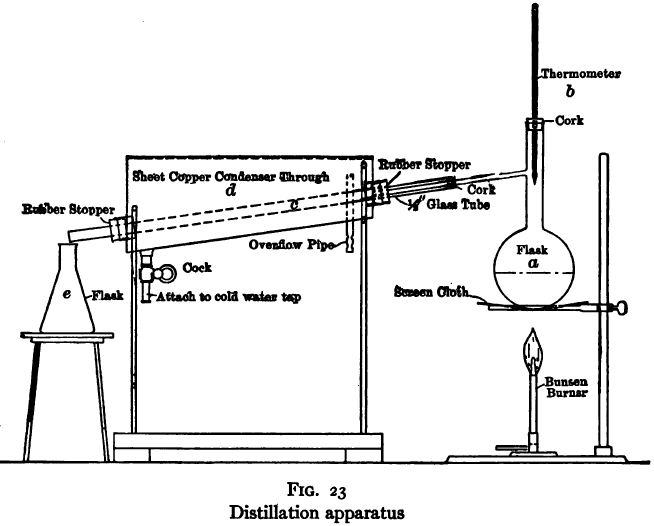
The purpose of the distillation test is to determine the percentages of the constituents of different boiling points. The apparatus for such a test is shown in Fig. 23. Place 500 gm. of the oil to be tested in the flask (a), connect with condenser tube (c) by means of a cork stopper, insert thermometer (b) in such a position that the top of the mercury bulb is on a level with the bottom of the side tube in the distilling flask, taking care that the bulb is in the center of the neck of the flask. Fill the condenser trough (d) with water. Place a weighed receiving flask (e) at the outlet of the condenser tube. Heat the flask (a) cautiously until such time as the water is completely removed from the sample. This time is indicated when bumping stops and when there is gentle ebullition of the boiling liquid. After the water is removed heat at such a rate that approximately two drops per second will fall from the condenser tip. The receiving flask should be changed at a temperature of 150° C. and at the end of each succeeding 50-degree rise. When the distilling temperature is above 250° C., the water in the condenser trough should be warmed to prevent solidification of the condensing material. Carry the distillation to a temperature of 350° C.
The interval of 50° C. in the end points of the various fractions is satisfactory for general rough distillation. If it is suspected that the oil is a particular substance, and a close analysis by distillation is desired, the points in the distilling scale will be chosen at intervals dependent upon the substance under investigation. For instance, with a tar or tar oil, the points will correspond to similar points in the manufacture of these oils. These points will be in general determined by specifications under which the oil is sold. Typical distillation analyses of common flotation oils are presented in Tables I to X.
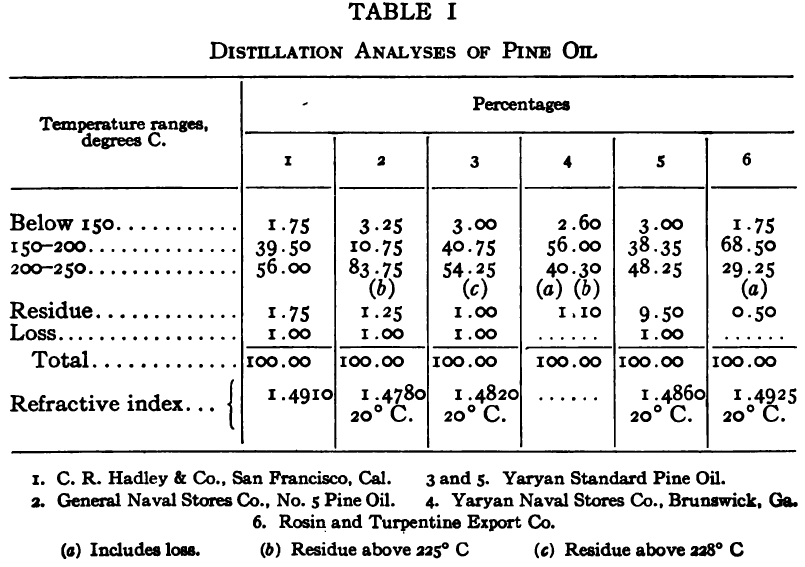
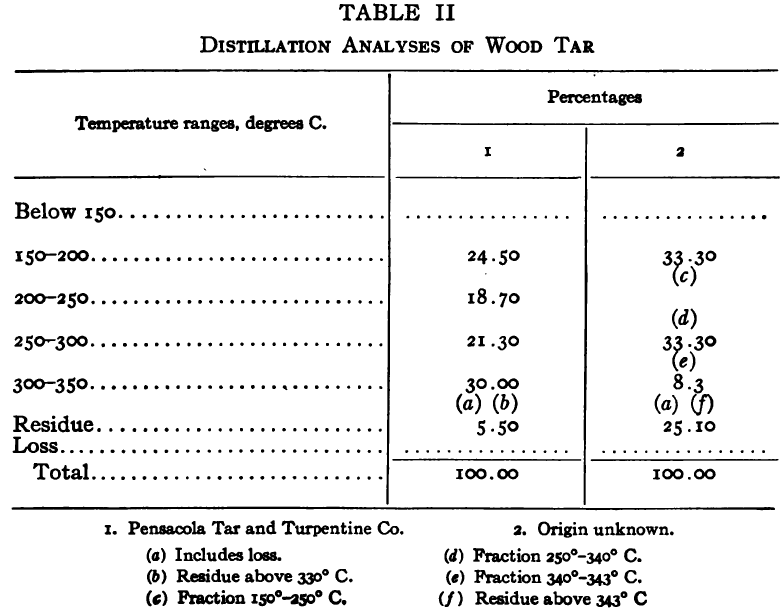
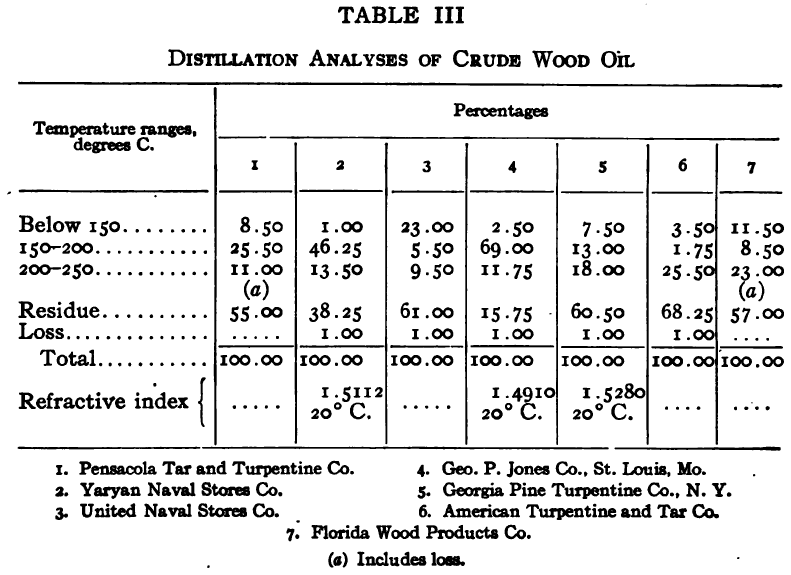
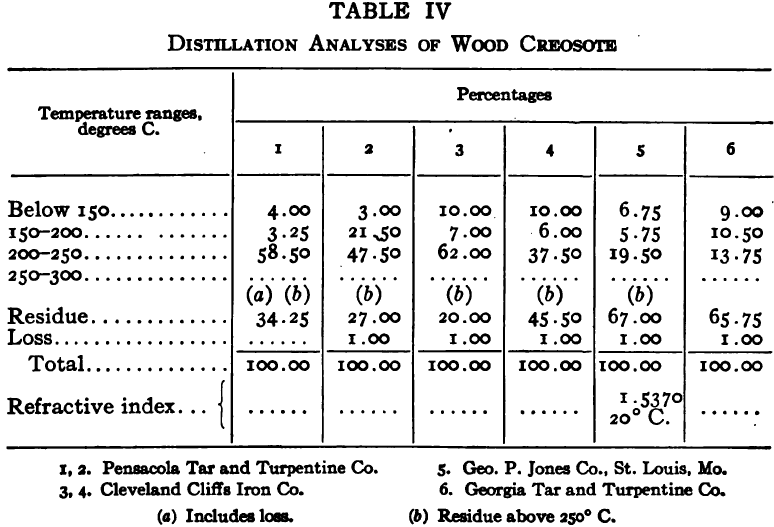
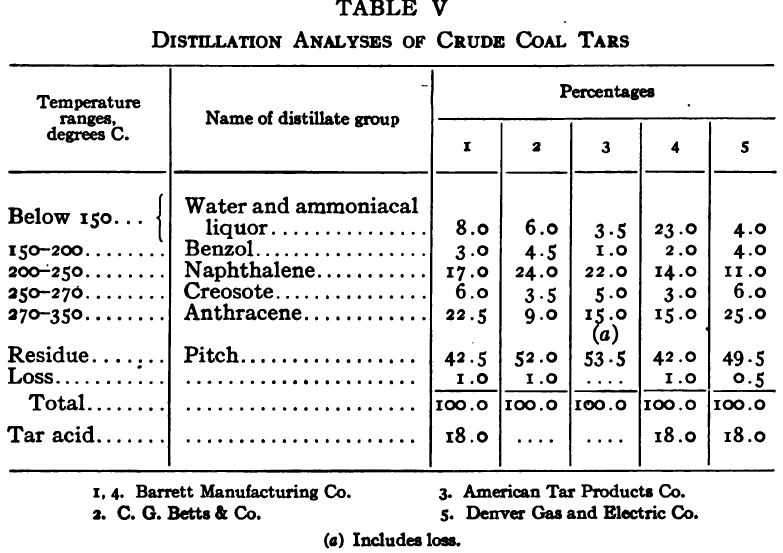
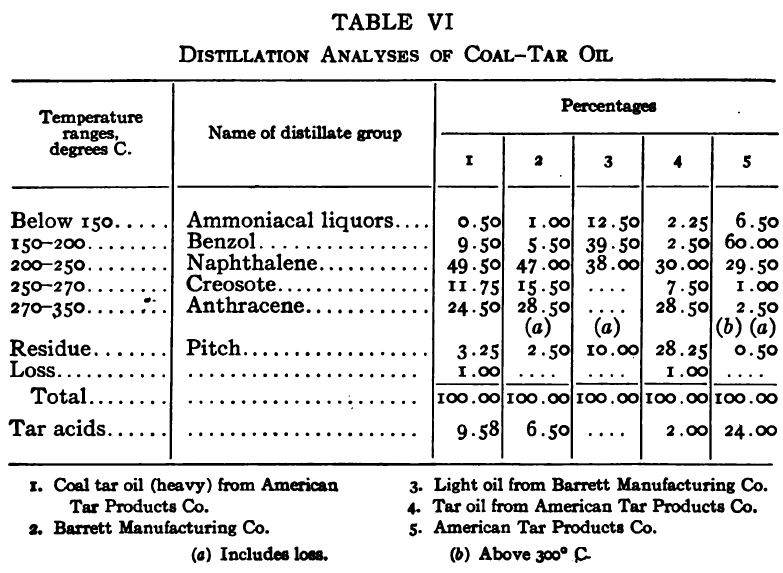
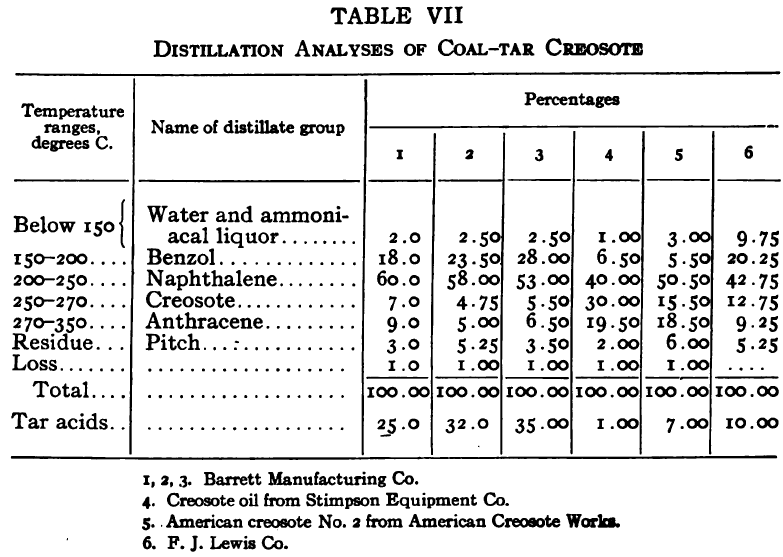
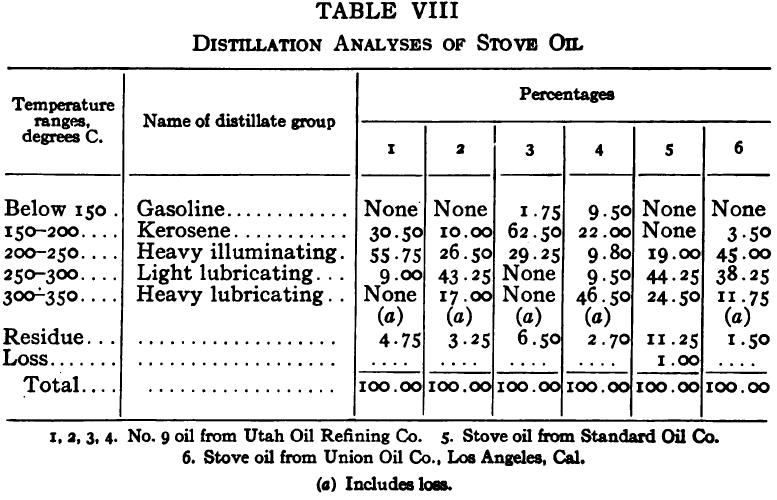
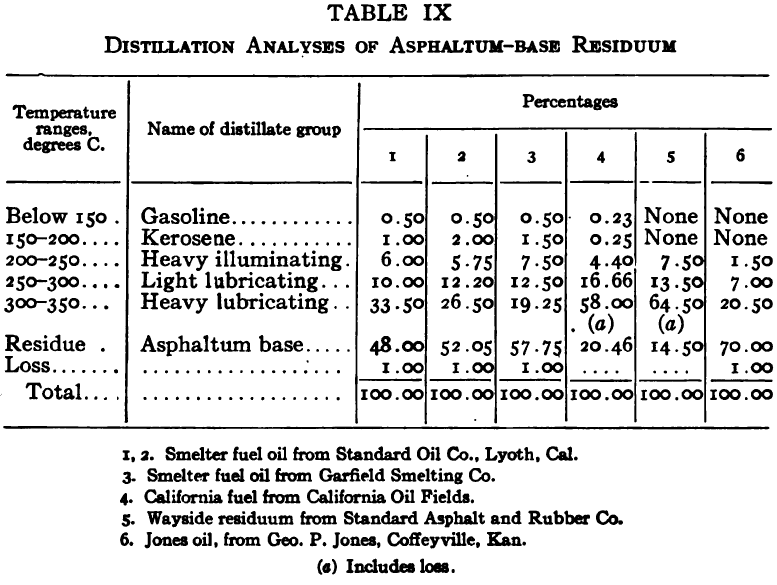
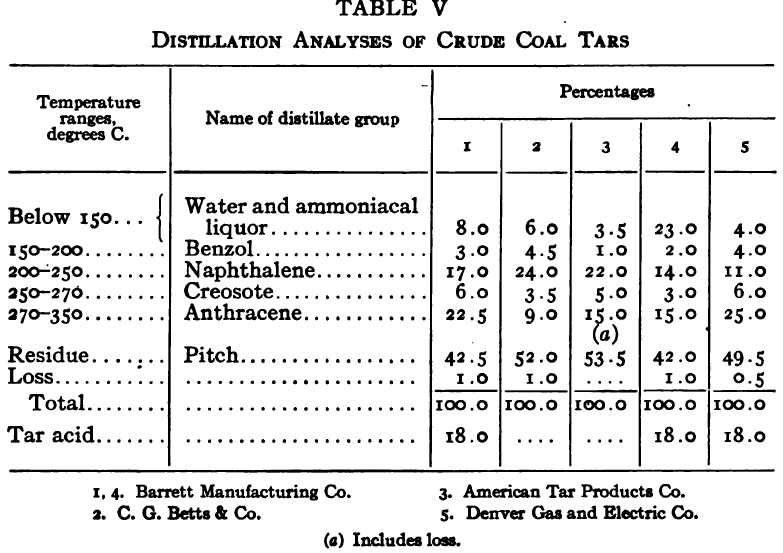
It should be borne in mind, as is shown by the tables, that a distillation analysis of one member of a given class of oils is not closely representative of all members of the class and that in flotation an oil of a given class may depart far from the distillation analyses herewith given, and yet serve its purpose in a wholly satisfactory manner. However, if a given oil is being bought regularly from a given manufacturer and the oil mixture in a flotation plant has been compounded and adapted to the needs of the plant, then any marked variation in distillation analysis is likely to be accompanied by a noticeable variation in the flotation results obtained by the oil mixture. It is possible, however, for a manufacturer to furnish oils of considerably different chemical constitution, which will give distillation analyses that check within the allowable limits established by practice, and where flotation results in a plant change with the initial use of a new lot of oil in the oil mixture, the composition of the oil should be investigated beyond the point possible by distillation analysis alone.
Refractive index
The velocity of light waves is different in different media. This fact causes light rays passing from one medium into another to be bent. The amount of bending resulting from the passage of a ray of light from air into a given substance is a specific property of the substance. It is expressed by the ratio between the sine of the angle of incidence and the sine of the angle of refraction. The angle of incidence is that between the incident ray and the perpendicular to the surface of the substance at the point of incidence. The angle of refraction is that between the same perpendicular and the direction of the ray in the substance. The index of refraction thus measured will be greater than 1.0 for all solid or liquid substances.
The index of refraction is determined by means of an instrument called a refractometer. The instrument is built in several different forms. The Abbe, which is a common form, consists of two prisms of dense flint glass mounted in water-jacketed frames for temperature control. These prisms are movable around an axis at right angles to the axis of the instrument telescope and are rigidly attached to a pointer which travels over a scale attached to the telescope. The pointer thus measures the angular relation between the prism faces and the telescope axis. Light entering the lower prism is in part totally reflected and in part refracted into the telescope. When the prisms are viewed through the telescope, therefore, a part of the field is dark and a part light. When the line of division between these two parts of the field is brought into coincidence with the junction of the cross-hairs of the telescope the pointer, in the usual instrument, reads directly the index of refraction of the liquid referred to air, at the temperature of the investigation. An explanation of the optics of the instrument is to be found in any good book on general physics.
Before determining the index of refraction of an oil, water should be passed through the circulating system and the plates brought to the temperature at which the determination is to be made. This temperature should be in the neighborhood of 25° C., but may be varied according to the consistency of the material under examination. When the desired temperature has been reached, clean the plates thoroughly with soft paper and ether or other suitable oil solvent. Place a drop of the oil to be examined on the horizontal plate, close the plates and lock by means of the locking screw. Allow time for the oil to attain the temperature of the plates. Now move the plates backward and forward across the line of sight of the telescope until the point is reached at which the division between light and shadow is coincident with the intersection of the cross-hairs. Read from the vernier the index of refraction of the oil. The determination should be repeated until concordant results are obtained. Read the temperature again and clean the plates with cotton and ether before setting the instrument aside.
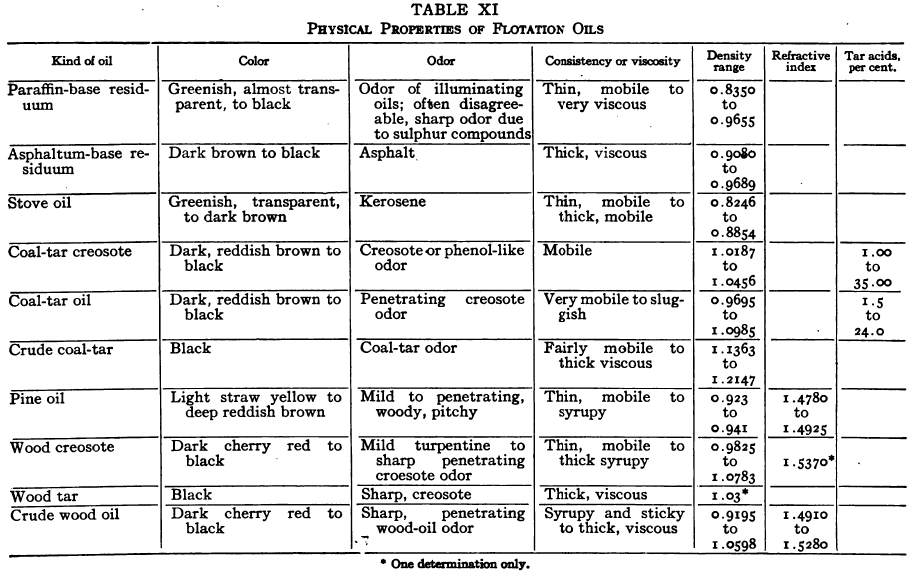
The refractive indices of several typical flotation oils are given in Table XI.
Odor
Smell is an important aid in the identification of an oil but characteristic odors cannot be satisfactorily described except against a background of experience. The experimenter should acquaint himself with the odors of the common classes of flotation oils as given in the list in Table XI.
Fluorescence
X-Ray is characteristic of petroleum products and will serve to indicate the presence of any considerable quantity of such products in mixtures.
Table XI presents certain physical properties of samples of some of the more prominent flotation oils. It will be seen that these properties vary markedly. Shipments of oil under the same name from the same dealer will vary considerably and shipments of supposedly similar oils from different dealers will vary widely. No definite relation between physical properties and usefulness in flotation has yet been worked out.
* See notes on page 70.
Oil Testing P. 125-145
