Many of our largest copper deposits contain both sulfide and oxidized copper minerals. The large porphyry mines, with a total copper content of from 1.3 to 2.0 per cent., send to their mills ore with an oxide content of from 0.2 to 0.4 per cent. While the recoveries of the sulfide minerals are considered satisfactory, using modern flotation practice, the recovery of the oxidized portion of the copper is small; better recoveries of oxidized copper were made before flotation methods were adopted.
Beside the ores containing copper mainly as sulfide, with a small amount of oxidized copper, many of the large porphyry mines have oxide orebodies, in which the proportion of non-sulfide minerals is so high that present methods of treatment are inefficient. In some of the well-known orebodies in the Miami-Inspiration district the copper occurs about equally in sulfide and oxidized forms; and the mixing of minerals is complete throughout the orebody. There is no possibility of mining the sulfides and the oxides selectively. At the New Cornelia mine, however, the oxide and sulfide ores are sharply separated, perhaps by an ancient water level, so that the oxide ore may be mined and treated in a plant built for that purpose, and the underlying sulfide ore may be left for treatment in another plant.
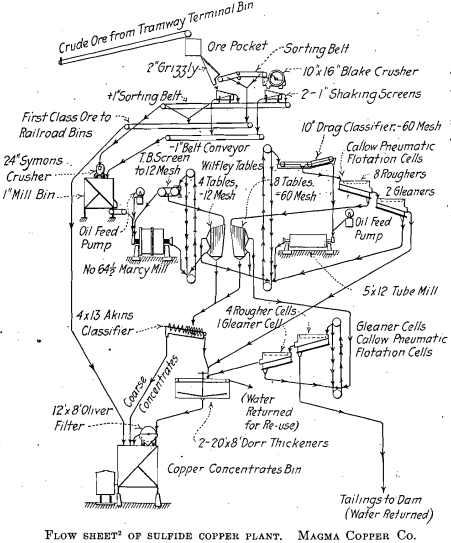
If copper is to be made from an ore having a total content of 1.5 per cent., it must be concentrated or removed from the ore by some hydrometallurgical method. Flotation is generally successful for the sulfide minerals and flotation after filming with sulfide seems to work in many cases on the pure carbonate minerals. Callow has given an extensive report on the results of “ sulfidizing ” flotation on the ores of the Magma mine. Where such a method can be applied, it may be the best solution of the mixed-ore problem, as it involves no solvent, no precipitant, no expensive plant, and no operation other than the one by which the sulfides are also concentrated. A comparatively small amount of an alkaline sulfide is used to film the carbonate particles with copper sulfide. The sulfide film is so thin that in many cases it is hardly visible and the carbonate concentrate comes off as green as the original minerals in the ore.
Unfortunately, much of the oxidized copper in the large porphyry mines is in the form of copper silicate. While it might not be very difficult to coat pure copper silicate with sulfide and recover it in concentrated form by flotation, concentration of most of the silicate copper of the Southwest would lead to nothing at all. The copper is in so dilute a solution that a bright blue color may mean a copper content no higher than 1 or 2 per cent. Furthermore, the density of this solution of copper silicate in silica is always less than the density of quartz; which means that gravity concentration, as well as flotation, leads to no result.
SULFURIC ACID AS A SOLVENT
Dissolving oxidized copper minerals in sulfuric acid is an ancient metallurgical method, but it is only within the past decade that this operation has taken a place in major operations of copper metallurgy. At the plant of the New Cornelia Copper Co., the oxidized minerals are dissolved from the ore with sulfuric acid in large vats, following crushing to about 3-mesh. The solution, containing copper, together with iron, alumina and the other impurities that dissolve from the ore, is passed through towers in contact with sulfur dioxide, to reduce the iron to the ferrous state. The solution is then sent to the electrolytic cells and refined copper is produced directly. After a part of the copper has been removed, the solution is sent back to the ore, with the addition of the necessary amount of “make-up” acid, and the cycle is continued.
Some of the solution, sufficient to cause the discard of the impurities dissolved each day from the ore, is removed from the cycle and passed over scrap iron. A corresponding amount of copper is produced in “cement” form, which is either returned to the main circuit by solution in the ferric iron or is, smelted.
If this method is adopted for most of the mixed ores, however, it will require a large plant for the recovery of the oxidized copper following 3-mesh crushing, the first cost of which, about $1000 per ton-day, must be charged to the oxidized copper recovered. While this charge might be reasonable for the New Cornelia plant, with a recovery of more than 25 lb. of copper per ton, it is a high charge for a mixed porphyry ore with a probable oxide copper recovery of 8 or 10 lb. per ton. Following the oxide treatment at 3-mesh fineness, the ore must be ground to flotation fineness and sent through the ordinary flotation operation.
If the ore were crushed to the fineness necessary for flotation, the oxidized copper dissolved in apparatus like that of a modern cyanide plant, the pregnant solution separated from the solids either by counter-current decantation or by filters, and the solids sent to flotation tanks, the saving in plant cost would be very large. While, this method involves many questions of acid-proof machinery, none seem difficult to answer in the light of common operations in many chemical plants.
The difficult part of this method often is the nature of the pulp, following treatment with acid. Many ores containing oxide copper are hard to settle or filter at flotation fineness as they come from the mine and they all are much worse after contact with acid. Settling areas of 40 or 50 sq. ft. per ton-day are not uncommon; for a 5000-ton plant, a small-scale operation, this would mean thickeners 400 or 500 ft. in diameter.
The effect of acid on the filtering qualities of some ores is very marked. Recently tests were made with an acid-proof vacuum filter on a mixed ore, ground to a size considered best to give high filter capacity and good washing efficiency. Before the ore was treated with acid, the filter had a capacity of over a ton per square foot per day; after solution of the copper, its capacity was about 300 lb. per sq. ft, per day. The filtration tests previous to acid treatment were with copper sulfate solution, instead of water, so that the washing could be tested on an actual copper solution.
The great change in filtering qualities was caused by the effect of the dilute acid on the solids of the ore, which was very siliceous, with not more than 5 per cent, of total soluble material that could be removed by the dilute acid. Many of the ores now waiting for a mixed-ore process are high in clay and talc and would be even more difficult to handle on filters than this one.
TREATMENT OF OXIDIZED COPPER BY AGITATION
Because of the difficulty of separating a pregnant solution from the solids of the pulp, five or six people, at about the same time, decided to grind to flotation fineness and dissolve the oxidized copper by acid in acid-proof Pachuca tanks, or some other form of modern agitator, precipitate the copper as metal, with fine iron, without any separation of solids from the pregnant solution, and float the precipitated copper and the sulfides together. Or, if the conditions of efficient flotation of metallic copper and the particular sulfides of the ore are not the same, float the sulfides out before the addition of the solvent acid, under whatever conditions best suit that operation; and float the precipitated metallic copper after precipitation in the pulp, under the necessary conditions for that flotation operation.
This plan has been tried many times during the past five years, in plants ranging from laboratory scale up to test operations of 100 tons per day. It appears to be feasible, but the nature of the ore must always be considered. Ore that has been out of the mine for some time is always refractory. Tailings, for the same unknown reason, are hard to treat in this way.
Early in the history of this process, it became evident that a special kind of iron would be necessary for precipitation in the pulp; sponge iron proved suitable for the purpose. The direct reduction of finely divided oxide of iron to metal, without melting, is an old process. It has been carried out in crucibles; in horizontal retorts; in modified reverberatory furnaces; in a rotary kiln of Bruckner type; in vertical retorts, and pipes. Sponge iron made by these methods is not “sponge” at all. There is no sintering together of the particles of metal, but each bit of oxide is changed to iron, and at the same time the surface of the particle is enormously increased; very much like the change that takes place in popping corn. Iron in this form seems to precipitate copper in a favorable form for flotation, and the development of the iron-reduction process seems to be the key to the “leaching-precipitation-flotation” operation.
SULFUR DIOXIDE AND AMMONIA AS SOLVENTS
Sulfurous acid, which is merely the solution of sulfur dioxide in water, is a good solvent for the oxidized copper minerals and has often been used. The mechanical difficulties do not appear insuperable. The solution of gas in water must be kept confined and applied to the ore in closed towers or, in the case of finely ground ore, in rotating barrels.

The first application of sulfur dioxide as a solvent for oxidized copper was by James W. Neill, at Butte, Mont., in the late nineties. Later, a patent was obtained by Neill and Burfeind, on a cyclic process, including the precipitation of a part of the copper from solution as cupro-cupric sulfite. The process was tried on a small-scale mill at one or two other places, but mechanical difficulties, apparently not connected with the chemical part of the process, prevented full commercial success.
During the past three or four years test plants have been running on sulfur-dioxide leaching, and while the large-scale questions have not been touched, the leaching results have been good in many cases. Sulfurous acid is much slower than sulfuric in the attack on the dilute copper silicate. Assuming complete success as a solvent, we still are confronted with the same difficulty. Shall we build the huge 3-mesh plant; can we separate pregnant solution from pulp; use fine iron precipitate in the pulp and float the precipitated metallic copper, either as a separate operation or with the sulfides?
It has long been known that ammonia, with the addition of an ammonium salt, such as the carbonate, is a good solvent for the oxidized copper minerals and for metallic copper. During the past five years, ammonia has been used successfully in the treatment of ores quite outside the range of any acid solvent. At the Calumet and Hecla mine, ammonia is used to dissolve native copper; at the Kennecott, it is used to dissolve oxidized copper minerals. At both places the ore is so high in calcite that an acid could not be used.
Theoretically, the process is perfect. After solution of the copper minerals, copper is precipitated as oxide by merely heating the solution. Precipitation is therefore a part of the normal step by which the leaching agent is recovered and sent back into the cycle. It appears from what has been published, that ammonia has been used as a solvent only in the treatment of coarse sands, where percolation in covered vats can be applied. Evident mechanical difficulties prevent its easy application to agitation-leaching of finely ground ore.
Where the ore carries a large percentage of acid-soluble impurities, the use of an acid leach is out of the question and the choice of a solvent is limited to ammonia, water (following a sulfating roast), and such solvents as ferrous chloride, which might be used if the percentage of soluble lime, etc. is not too high. The ammonia process is quite sure to find other applications than the two mentioned.
IRON SALTS AS SOLVENTS
Many of the processes proposed and patented during the past 20 years depend on the solvent action of salts of iron. The basic principle of all the iron processes is the slight solubility of ferric hydroxide, and the effectiveness of the iron salts is measured largely by the degree of hydrolysis they show. This means merely that an iron salt in solution is a reservoir of dilute acid, and that as fast as this acid is consumed, more is formed, at the same time that ferric hydroxide precipitates.
The most extensive leaching operations in the world are being carried on with the iron salts; these are the great heap-weathering operations at Rio Tinto, Tharsis, and some of the Russian mines. Oxidation of the sulfides by ferric sulfate and solution of the oxides by sulfuric acid, also formed from ferric sulfate, are the reactions that take place.
Ferric sulfate (or even better, ferric chloride, where salt is easily available) has many possibilities in connection with a mixed copper ore. It will attack and oxidize the sulfides and will dissolve the oxidized minerals and the artificial oxides resulting from the attack on the sulfides. If an unlimited supply of cheap ferric sulfate were available, the mixed-ore problem would be quickly solved.
OTHER SOLVENTS FOR OXIDIZED COPPER
An incomplete list of processes and patents mentions at least 20 solvents, simple and complex, which are proposed as leaching agents. They range from nitric acid, which is doubtless good but rather expensive, to sodium carbonate and the caustic alkalies.
Under proper temperature control, a mixed ore containing sufficient sulfides, or one to which pyrite has been added, can be roasted to a calcine in which practically all the copper is soluble in water. The temperature must be high enough to cause reaction between the copper minerals, the sulfur of the sulfides, and the oxygen of the air; it must be kept below the point at which the copper sulfate is decomposed. This means that the furnace must be kept within the range 500° to 600° C. At a lower temperature, the reaction is slow and incomplete; at a higher temperature, the copper sulfate is in part decomposed to oxide and acid must be used to dissolve it.
To be efficient, sulfating roasting should give satisfactory commercial recovery of the copper of the ore with water alone, without the necessity of using any acid. A true sulfation of copper is accompanied by reactions that make many of the other constituents of most ores acid soluble. Often as much acid is consumed in dissolving the last 15 or 20 per cent, of the copper as is necessary to dissolve all the copper, following a “dead” or oxidizing roast of the same ore.
CONTROL OF TEMPERATURE DURING SULFATION
The attempt has frequently been made to control temperature during sulfation so that the iron sulfates are decomposed and the copper is water soluble. To accomplish this, the temperature is raised to just above 600° C. toward the end of the roast. In this way, it is possible to make the larger part of the iron of the calcine insoluble in water and slowly soluble in dilute acid; but this seems often to be accomplished at the expense of water-soluble copper.
The most accurate control of temperature during roasting is attainable in a muffle furnace, with which the best results in sulfating roasting have been obtained. During the past four or five years, encouraging results have been obtained by the use of outside combustion chambers, from which hot gases are sent into the furnace proper.
Whether copper sulfate is formed from a copper mineral, the sulfur of a sulfide, and air oxygen, or whether it requires the interposition of a sulfate of iron for its production is not clear. Certainly sulfating roasting with the addition of ferrous sulfate to the charge is easy and rapid; it is quite probable that the sulfates of iron play an important role in any efficient sulfating operation.
While so far all sulfating roasting has been on material that was not self-roasting, there is no difficulty about adapting the principle to ores high in sulfur and in getting high water solubilities from such material without the use of any external fuel whatever. Such, a method offers an interesting means of getting copper into solution, as the only expense involved in the operation is the charge against roaster operation and the leaching of the calcine with water.
THE PRECIPITANT
After expending money in getting copper into solution, we are faced with the equally difficult and expensive operation of getting it out again. The oldest and most common of the precipitants is iron, usually applied in the form of pig iron or scrap; shot iron is used occasionally, in tumbling barrels. It is not easy to make a neat job of this precipitation.
ELECTROLYSIS
Contrary, to the predictions of eminent men in the profession, good electrolytic copper is being made from leach solutions coming directly off the ore. The difficulties in doing this at the plant of the Chile Copper Co., where chlorides and nitrates are dissolved from the , ore, seem to have been successfully overcome. The difficulties at New Cornelia are of more immediate interest in this connection, as the oxidized portion of the New Cornelia is much like the oxide ore in other mines where a mixed-ore treatment is being considered.
The successful production of electrolytic copper, at Ajo depends on control of the ferric iron in the solution entering the tank house. A good deal of iron, in both ferrous and ferric forms, is dissolved from the ore. More ferric is produced from ferrous during electrolysis, and there is very little reduction of iron during leaching. If satisfactory power efficiency is to be maintained in electrolysis, the iron must be reduced artificially, which is done by saturating the nearly neutral solution, coming from the ore, with sulfur dioxide. Iron is reduced to the ferrous form in this way and a corresponding amount of sulfuric acid results from the reaction.
It may be taken as a general statement that anyone considering the making of electrolytic copper from leach solutions should look carefully at the question of iron dissolved from the ore. In the Miami-Inspiration district the mixed ores are highly siliceous and give up much less iron when treated with acid than do the ores of Ajo. It is probable that the Miami-Inspiration ores can be leached: and the solutions used for making electrolytic copper with less difficulty than has been experienced in the Ajo operation. It is certain that there are many ores to which the standardized New Cornelia operation can be applied for the recovery of the oxidized copper. As has been already explained, the question of first cost of plant is the hard one to answer. Metallurgically, the problems seem to be solved.
SULFUR-DIOXIDE PRECIPITATION
If a 2 or 3 per cent, solution of copper as sulfate is saturated with sulfur dioxide in a closed vessel, and heated to a temperature of about 150° C., approximately one half of the copper is precipitated as crystalline metal, with regeneration of a corresponding amount of sulfuric acid. For many ores, the amount of acid made in this way would be sufficient to dissolve the soluble copper and no acid need be brought in for the purpose. The method is not new. The first patent covering it expired in 1921, but so far as I know, it has not been used on a commercial scale.
The full-scale application may not be as easy as the description would indicate. The heating of a dilute copper solution means the expenditure of much fuel and fuel efficiency means, in this case, careful heat interchange between solution going to precipitation and solution returning from it. The vessels in which copper is precipitated must be large if the plant is to turn out 75,000 or 100,000 lb. of copper per day. The sulfur dioxide used in the precipitation must be pure; this means a large plant for making pure sulfur dioxide from roaster or flue gases.
No data on plant or operation costs have been made public, but it seems very likely that the total investment for a plant for sulfur-dioxide precipitation, including the plant for making the pure gas, will not be much less than for making electrolytic copper; the operation cost per pound of copper will be about the same for the two methods. In either case, the cyclic operation cannot be kept closed. A certain volume of solution must be regularly removed and “discarded” to obviate the accumulation of soluble substances from the ore. This “discard” must be “cleaned up” in a plant set aside for that purpose, either by precipitation of the copper on iron, or, if the amount of iron dissolved from the ore is small, by electrolysis. This applies as well to precipitation by sulfur dioxide as to the making of electrolytic copper.
HYDROGEN SULFIDE AS PRECIPITANT
The use of waste sulfur dioxide is invariably suggested when one considers the smoke escaping from the stacks of a neighboring smelter; equally suggestive is the possibility of utilizing other smelter products as adjuncts to hydrometallurgical processes. Hydrogen sulfide can easily be made from low-grade copper matte, or from matte made specially for the purpose. It might well be used as a precipitant for copper, either from a clear solution or in a pulp, following solution by acid, and preceding flotation, either of the precipitated sulfides alone or together with the natural sulfides of the ore. In either case, precipitation of copper from sulfate solution by hydrogen sulfide results in the regeneration of an equivalent amount of sulfuric acid, and, if the operation takes place in a clear solution, this acid can be used in cyclic operation to dissolve soluble copper minerals from the next batch of ore. As far as I know, this method has been tried only on a test-plant scale of a few tons of ore per day and the results seem to indicate that the expense of the operation, in most cases, will be greater than for precipitation on iron. It seems to be an operation that might advantageously be combined with smelter operation in some cases. It probably has just about the same number of difficulties as any other process of kindred nature.
PRECIPITATION BY HEAT ALONE
In the ammonia process, during the distillation and recovery of the solvent, copper oxide is dropped out; in the Neill sulfur-dioxide process, raising the temperature of the pregnant solution drives out sulfur dioxide and causes the precipitation of about 75 per cent, of the copper as cupro-cupric sulfite. Precipitation in the ammonia process is complete when all the ammonia is distilled out of the solution. In the Neill process, it is necessary to clean up the solutions with iron or by electrolysis, or with some other precipitant, to get the last of the copper.
OTHER PRECIPITANTS
The suggested and patented precipitants range from metallic lead to sawdust and from bleaching powder to carbon monoxide gas. Both lime and limestone have been used in extensive tests but neither, so far as I know, in regular commercial operation. Either hydrogen or carbon monoxide will theoretically reduce copper to metal, but practically the action is so slow that it cannot be used. Ferrous oxide would be a good precipitant, but it seems to be harder to make than iron and is not as good.
MECHANICAL HANDLING OF ORE AND SOLUTION
Practically all the problems connected with the acid-proofing of a leaching plant for sulfuric acid have been solved at Anaconda or at New Cornelia. Far more difficult problems, involving the very corrosive solutions resulting from the action of sulfuric acid on their special ore, have been solved at Chuquicamata, by the Chile Copper Co. The ammonia process offers no difficulties in the line of structural materials. The entire plant can be of iron. The solutions resulting from sulfur- dioxide leaching are of much the same nature as from sulfuric acid.
There remain the questions covering contact of solution with ore; the separation of solids from pregnant solution; thickeners; filters; efficient precipitating machinery for use with iron, etc.
Based on the successful operation of the Spanish heap-weathering methods, a number of large-scale tests on the heap leaching of mixed ores of copper have been begun in the Southwest. The method is of great interest to many mines, as very low-grade ore, practically waste, stripped from higher grade bodies can perhaps show a profit by this treatment. It is evident that the reactions in these heaps will not be quite the same as in the oxidation of the clean pyrite ores at Rio Tinto, because of the presence of considerable oxidized copper, which will rob the solution of acid during the first stages of the leaching operation. It is quite probable that the oxidized portion of the copper will be largely leached out before the sulfides are attacked.
In these operations the precipitant is always iron, and its use appears to be necessary, as the, returned barren solutions from which copper has been precipitated with iron are the active reagents of the process.
Heap weathering and sulfating roasting in a special furnace have been tested on a large scale, at the Shannon Copper Co., on ores with a content of soluble impurities so high as to preclude the application of an acid leach. Leaching was with water and with the iron- salt solutions resulting from precipitation on scrap iron; extractions by both methods were encouraging.
ADVANTAGE OF SULFIDIZING FLOTATION
Where the oxidized minerals in a mixed ore consist largely of the carbonates and cuprite, a filming treatment and “sulfidizing flotation” appears to offer the greatest advantage. Both sulfide and oxidized minerals are recovered in the same concentrate and sent to the smelter together. No large plant is necessary and there is only a small expense for extra supplies, compared with regular flotation operation. There will undoubtedly be difficulties with the flotation operation and the final recovery of oxide copper may not be so high as with an efficient solvent, but, with favorable ores, it is hard to see how anything can compare with it.
On many of the porphyry ores, the recovery of oxide copper by this method would be very small; or, if some way were found to float the silicate copper, the grade of concentrate would be lowered to a point where smelting costs would be prohibitive. From most of these ores, the oxide copper must apparently be dissolved by some solvent.
The oxide orebodies, in which the copper is present about half as sulfide and half as oxidized minerals, and where the recovery to be expected is 25 or 30 lb. per ton of ore, might well be treated first by the method used at New Cornelia, to recover the oxide copper, then reground and sent through the regular flotation operation.
The per-ton charge for interest and amortization, to be charged against, say, 15 lb. of copper recovered in the oxide plant, might be 30 cents. While two cents per pound additional cost at this point is a heavy burden, it may be accepted with the certainty of a standard operation. It must be remembered that the interest and amortization charge for any type of oxide plant will be high per pound of copper, on these ores. For the recovery of the oxide copper from the regular milling ore of the porphyries, or from the tails sent out from their mills, some other plan would meet with a heartier welcome. A charge of 30 cents against 6 or 8 lb. of copper is too high. For these reasons, the suggested process of leaching-precipitation-flotation is of special interest. The additional plant necessary is comparatively inexpensive, probably not more than a third as much as for the 3-mesh leaching and electrolytic copper.
The differences in operating cost between sulfuric acid and sulfur dioxide, electrolysis and iron precipitation, or precipitation by sulfur dioxide, cannot be estimated for these ores with any great accuracy. It is not likely, however, that the final estimates would show great differences in any of these combinations. I am inclined to feel that decisions regarding the process to be adopted will turn largely on questions such as certainty and fool-proofness and the availability of standardized methods of handling and treatment.
From the general data at hand, a reasonable forecast of what may be expected to occur might be:
That the oxide orebodies may be treated as at New Cornelia for extraction of the soluble copper; then reground and sent through regular flotation treatment.
That some ores, with a rather high content of soluble copper, may be treated by leaching-precipitation-flotation.
That some low-grade ores will be successfully treated by heap leaching.
That sulfating roasting will be used at favorable points both for the treatment of mixed ores containing a high, percentage of soluble impurities and for the treatment of low-grade concentrates high in pyrite.
