The present is to discuss some of the current silver-treatment plants and also reviews briefly some of the older practices in important silver-mining areas since closed down. The greater part of the world’s production of silver is derived from the refining of the base metals, particularly lead ores, and complex ores of lead, copper, antimony, and zinc. Most of these ores are concentrated by flotation methods, and the concentrates smelted.
There are silver ores, however, where the base-metal content is too low to justify the above conventional form of treatment, and cyanidation offers the most economic recovery method.
The previous pages have been devoted to the treatment of gold and silver ores in which the recovery of silver, because of the relatively small amount present, is not ordinarily of economic importance. There are, however, certain mining areas where the recovery of the high silver values is or has been the principal metallurgical problem.
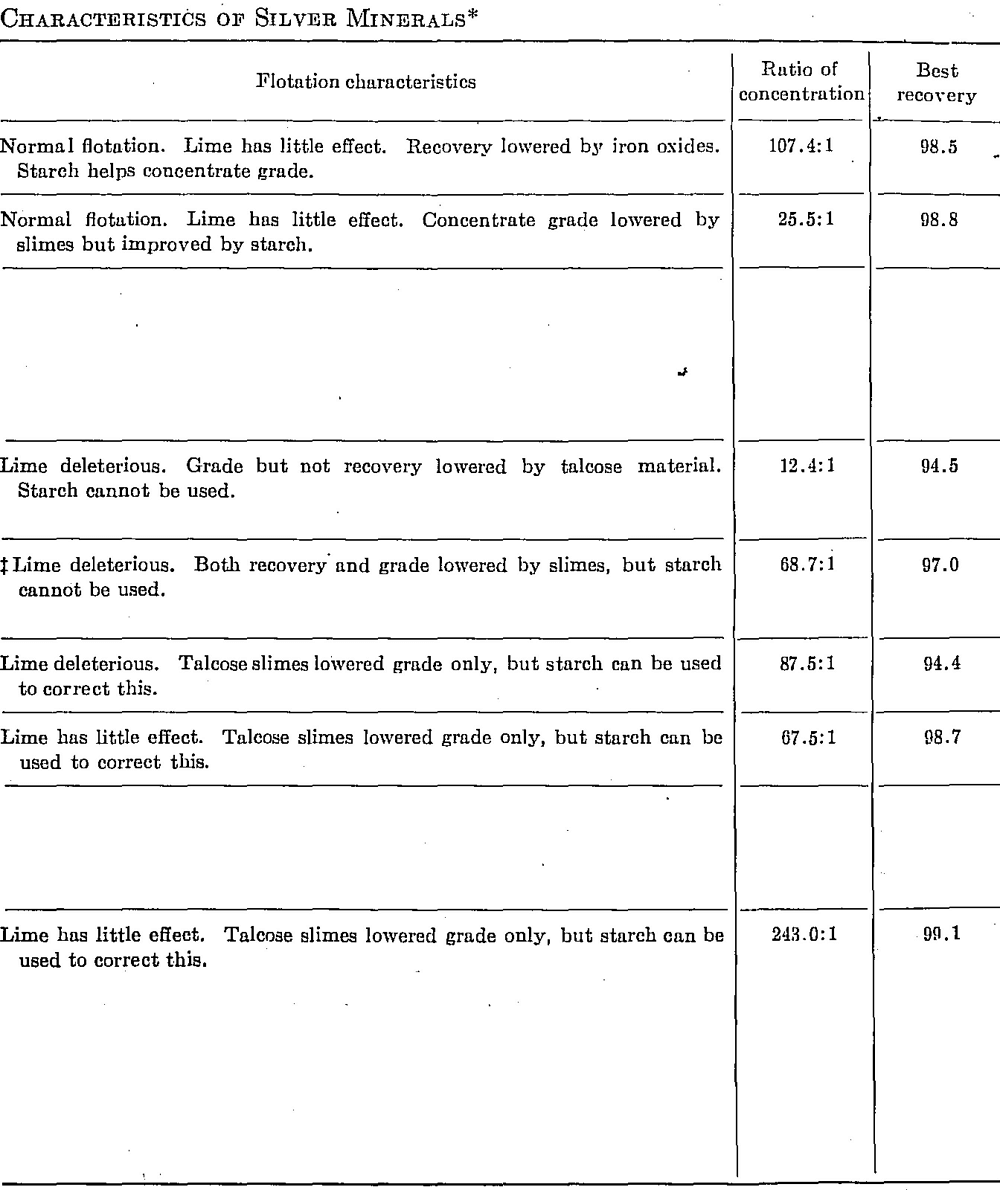
Table 91 gives some of the more important cyanidation and flotation data obtained in laboratory tests on relatively pure samples of silver minerals.
NATIVE SILVER
The ores of the Cobalt area were remarkable for their high content of silver and for the complex assemblage of minerals found in the veins and enclosing rock. Of the silver-bearing minerals, native silver was of outstanding importance, as fully 97 percent of the values occurred in this form. It was found in masses ranging from large slabs to the finest, filmy leaf. Other minerals included cobalt and nickel in the form of arsenides, sulphides, antimonides, and various combinations of these, associated and often intimately mixed with a number of base-metal compounds.
A variety of methods were used for treating high-grade ore and concentrates, including the amalgamation and cyanide process, the hypochlorite-cyanidation process, the sulphuric acid-cyanidation process, and chloridizing roasting, while the lower grade material was treated by a combination of gravity concentration and flotation or cyanidation.
SILVER SULPHIDES
The ore was oxidized and siliceous, the principal constituent of the gangue being quartz with some calcite. The silver minerals contained in the ore were principally argentite and cerargyrite, the former predominating. The lead minerals, all of which were argentiferous, were chiefly cerussite and galena, with occasionally a little anglesite. The gold was free, but most of the ore contained merely a trace.
The milling scheme included tabling at 10 mesh followed by regrinding to 80 to 90 per cent minus 200 mesh and cyanidation by Pachuca agitation. The solutions were maintained at 2.8 to 3.2 lb. per ton NaCN, and the reagent consumption was 6 lb. lime, 2.5 lb. NaCN, and 0.25 lb. zinc dust per ton of ore. Table concentrates averaged about 426 oz. silver per ton and carried 52.5 per cent lead. The zinc precipitate analyzed 20,000 oz. silver and 5.60 oz. gold per ton and carried 1.02 per cent zinc and 24.1 per cent lead. Both products were shipped to Carteret, N. J.
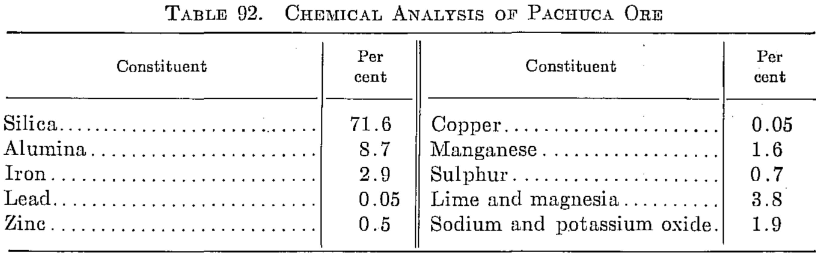
Silver in the Pachuca district, state of Hidalgo, Mexico, occurs chiefly as argentite. A part composite analysis of ore treated at the present time is given in Table 92.
Flotation has been given thorough trials but has not succeeded in equaling the economic results of cyanidation, according to R. R. Bryan and M. H. Kuryla in Trans. 112, A.I.M.E., 1934.
The Loreto plant of Compania de Real del Monte y Pachuca has a daily capacity of 3800 tons and is the largest silver-cyaniding works in existence. No concentration is done.
The property was purchased by the Mexican government from the United States Smelting, Refining and Mining Co. in September of 1948. For several years past the tonnage and grade of ore has been dropping and now stands at about 100,000 tons per month, assaying 300 grams silver and 3 grams gold per ton. The higher ratio gold than previously is due to the discovery some 5 years ago of a new vein carrying about 10 grams gold per ton.

Abstracted from: “Oxygen as an Aid in the Dissolution of Silver by Cyanide, “R.I. 3064, U.S.B. of M. and “Flotation of Silver Minerals,” R.I. 3436, U.S.B. of M. Ag dissolves according to the reaction:
AgCl + 2NaCN = NaAg(CN)2 + NaCl
Where argentine is intimately mixed with pyrite, sphalerite, and gangue, roasting for 1 hr. up to 460°C was necessary for a +75 per cent extraction. Time is the important factor for argentite when pure. If temperature of roast exceeds 600°C an insoluble silver silicate is formed.
In the case of polybasite, silver may be partially replaced by copper and antimony partially replaced by arsenic.
Not all samples of tetrahedrite are as refractory as the one tested. Some yield up to 83 per cent extraction.
In general the treatment includes:
- Fine grinding.
- High alkalinity.
- Lead and mercury salts to precipitate alkaline sulphides.
Tests showed that:
- Oxygen was an aid in dissolving silver minerals.
- Warm solution are an advantage.
- A low- temperature roast gives the best result of all.
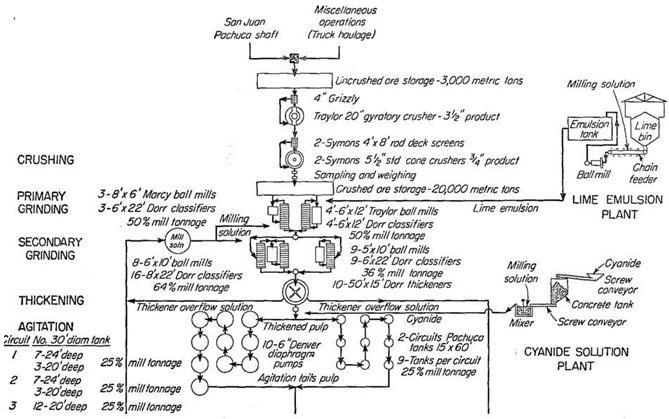
Some details of 1948 operations of the Loreto follow (see Fig. 95):
Crushing Silver Ore
Mine ore of a maximum size of 12 in. is reduced to 7/8 in. by one gyratory and two cone crushers, between which are grizzlies and vibrating screens.
Grinding Silver Ore
Two-stage grinding in cyanide solution is practiced. For primary grinding, 8- by 6-ft. Marcy grate mills and 6- by 12-ft. trunnion Traylor ball mills are used in closed circuit with 6- by 22-ft. Dorr classifiers, which are the only type operated in Pachuca. Nearly 80 per cent of the feed to these mills is coarser than 3 mesh and up to 1 in. The classifier overflow is 64 per cent solids. For secondary grinding, 6- by 10-ft. Traylor mills of the trunnion type and 5- by 10-ft. trunnion mills of local make are used in closed circuit with an 8- by 22- and a 6- by 22-ft. classifier, respectively. The classifier overflow contains 20 per cent solids; a sieve test of the final product shows on 48 mesh 2.20 per cent; on 65, 7.90; on 100, 9.17; on 150, 13.13; on 200, 8.09; and through 200, 59.51 per cent.
Thickening
Ten Dorr thickeners, 48¾ by 15¼ ft., yield a pulp of 45 per cent solids.
Between 1934 and 1945 the ore became more difficult to settle and underflows dropped to as low as 30 per cent solids. The trouble was largely overcome by removing about 1000 tons per day of plant solution and replacing with fresh cyanide solution. The solution removed is plant barren solution, which is first passed through the regeneration plant to recover its cyanide, silver, and gold content and then discarded with the tails.
Silver Ore Agitating
Eighteen Pachuca tanks, 15 by 60 ft., and 32 “flat” tanks, 20 and 24 by 30 ft., do the agitating. The latter is a tank equipped with a Dorr-thickener mechanism and air jets. Air at 35 lb. pressure is used in the Pachucas and at 18 lb. in the flat tanks. Agitation proceeds, for 73 and 70 hr., respectively.
Aero-brand cyanide is dissolved in barren solution to make a strong solution, and this is added to the agitators to bring the strength to 0.17 per cent NaCN. Litharge is added in the dissolving tank to eliminate soluble sulphides. Cyanide consumption, excluding regeneration, amounts to 1.62 kilograms per ton ore. Lime consumption is 9.0 kilograms.
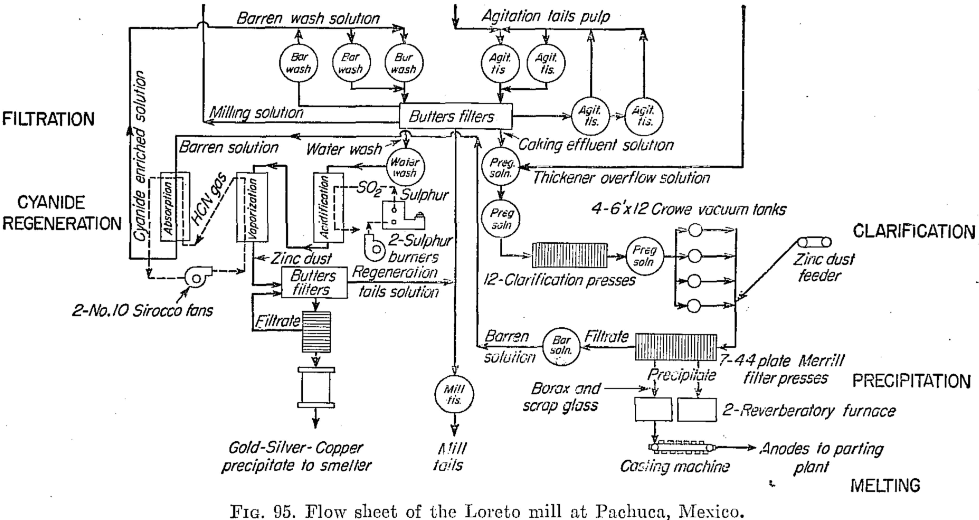
Filtration
Butters tanks, each with 187 leaves, 67 by 117 in., do the filtering. Each tank averages 11 cycles of 128 min. each day, and each cycle is divided into 26 min. for caking, 38 min. for barren wash, 15 min. for water wash to mill, and 20 min. for water wash to regeneration, the remaining 29 min. being required for filling transfers, discharging, etc. A vacuum of 18 in. is maintained. Average cake is 7/8 in. thick.
Clarification and Precipitation
Solution from the filters is clarified in 12 Sweetland presses, which can handle 2¼ tons per day per square foot of surface. They are discharged twice and cleaned once each day, and leaves are acid-treated every 10 days.
The Merrill-Crowe system of zinc-dust precipitation is used. Centrifugal pumps force the solution through the presses. Zinc consumption is 170 grams per ton of ore. The dried precipitate assays 83 per cent silver and 0.46 per cent gold, also 0.25 per cent selenium and some other metals.
Melting and Refining Silver Ore
Precipitate is melted to bullion in the usual manner, granulated borax and bottle glass being used, in an oil-fired reverberatory furnace of 15 tons’ capacity. The temperature is raised to 1050°C., and slag is skimmed off. Air is then blown in, and the slag is skimmed for 60 hr. Then the metal is tapped into a continuous anode-casting machine. Anodes weigh 10 kilograms, and a furnace charge makes 2000 of them in 5 hr. of casting. The bullion is increased in fineness from 950 to 993; copper is the principal impurity remaining.
The anodes are next parted in 200 Thum-type electrolytic cells, and the resultant silver is 999 plus fine. The gold mud is reduced to anodes, which are treated in Wohlwill cells, giving gold 999.8 fine.
Extraction
The current extraction (1948) now averages 85 per cent of the silver and 90 per cent of the gold contained in the ore.
Cyanide Regeneration. The plant for the regeneration of 3800 tons of cyanide solution per day is described earlier.
Treatment of Silver Ores at Tonopah
The old milling practice used at Tonopah, Nev., well-known silver district, is of interest to metallurgists today because of certain special treatment features discussed below.
In Tonopah there are five mills: the Belmont, Extension, MacNamara, Montana, and West End, while at Millers, 12 miles north, are the Belmont and Tonopah mills, ore being shipped to these at a cost of 70 cents per ton. In nearly every case gyratory crushers are used for breaking ore as it comes from the mines, the procedure being to crush first in a large crusher up to the No. 7½ type K Gates size and pass through revolving trommels, the oversize being again reduced in No. 3 size gyratories, the final product for the stamps being about 1¼ in. Sorting is done at the Belmont and MacNamara mills, at the former on a pan conveyor from which 15 per cent is rejected, and at the latter on a 30-in. rubber belt from which 6 per cent is sorted out. From the crushing department, the ore is taken to mill bins by 20-in. belt conveyors, or bucket elevators, and distributed by the usual automatic devices.
There is no amalgamation at Tonopah, nor is it necessary on this class of ore. Crushing is done in weak and warm (from 50 to 80°F.) cyanide solutions, so the ore is in contact with solution from the stamps to filtration. This is necessary as well as the heating, which, although somewhat expensive, quickens the solution and accelerates the dissolving action. Solutions are usually heated to about 95° and in one case to 120° by live steam introduced in the agitators.
The practice of using hot solutions is briefly as follows: At the new Belmont mill the temperature at the stamps is from 60 to 70°F., and at the Pachuca agitators exhaust steam from the mill air compressor is fed in, increasing it from 90 to 100°. In the M. and S. Press of Jan. 27, 1912, A. H. Jones, metallurgist at this plant, gave some valuable data on this subject. On an ore carrying 0.05 oz. gold and 18.2 oz. silver per ton, 60 hr. agitation with both 60 and 90° solutions, the tailing averaged
0. 0175 and 3.45 and 0.0125 and 1.90 oz., respectively. Tests on 48 and 69 hr. at similar temperatures gave as marked results. Besides the effect on extraction, the hot solutions flowing through the mill kept the whole place at a good working temperature. At the Montana-Tonopah, ore is crushed in 50 to 60° solution, which is increased to 110° at the Hendryx agitators by live steam. It is found also that the heat aids settling. There is a marked decrease in extraction without hot solutions.
Tonopah ores carry as much as 3 per cent pyrite, but concentration is not always employed, it being done only at the Belmont, Montana, Tonopah, and West End. It would seem that, if the grade of the ore and percentage of mineral are not too high, tables are not necessary, and this varies from time to time in the various plants. At any rate, a very close saving is not attempted. The Extension Company dispensed with their Deister tables, selling them to the West End. The Belmont, Montana, and Tonopah use Wilfley tables. Concentrate is collected, steam dried in large trays, sacked, and shipped to smelters. Freight and treatment cost nearly $70 per ton.
All-sliming is the standard method, with the exception of the Tonopah mill at Millers, where three products are made: concentrate, sand, and slime. At this plant reduction is by stamps and Chilean and Huntington mills, while at Tonopah the procedure is as follows: The pulp from the stamps is fed into Dorr duplex classifiers making 12 strokes per minute, from which slime overflows and coarse material is fed into tube mills by means of a special feeder. Discharge from these is elevated to the Dorr classifiers, where a further classification takes place, followed by further grinding in the tube mill, and so on.
Various types of thickeners or dewaterers are in use, the practice being to allow the clear solution to overflow and decant off as much as possible for battery storage. When it gets too high in gold content, it is decanted to the tank for precipitation. As at many other mining centers there is quite a difference of opinion regarding the efficiency of agitators, the Trent being used at the MacNamara, Montana, and West End; the Hendryx at the Montana; Pachuca tanks at the new Belmont mill; and ordinary mechanical agitators and air lifts at the Belmont and Tonopah at Millers, these being in series at the Belmont plant. Centrifugal pumps and air at about 20-lb. pressure are used for the Trent system, and better results are obtained if pulp is drawn off near the top of a full vat and pumped through the arms as usual. Agitation proceeds for upward of 48 hr. At the new Belmont mill, slime is first agitated in six Pachuca tanks, and from these it is elevated to Dorr thickeners by an air lift, prior to going to another set of six Pachucas, making a total of 48 hr. agitation, the idea being to get rid of as much valuable solution as possible before sending slime to the filter plant. Cyanide and lead acetate are added to the agitators, the former being from 2 to 5 lb. solution, while regular addition of the acetate is found necessary at all mills. Lime is usually slaked and added to the tube-mill feed. Consumption of chemicals at the Extension is as follows:
Lead acetate……………………………….0.9 lb. per ton
Cyanide…………………………………….2.5
Lime……………………………………….3.5
Agitated slime is drawn off to stock tanks, which serve the purpose of storage from agitators and excess from filter plants. The latter have little of special note about them, being of the ordinary stationary leaf type which has been described so often in technical papers.
Zinc-dust precipitation is used at the new Belmont and Montana mills, and zinc shavings at the Belmont, Extension, MacNamara, Tonopah, and West End. Methods of dealing with precipitate vary somewhat. At the new Belmont precipitate is dried, mixed with 5 per cent borax, and smelted in double-compartment, oil-fired Rockwell furnaces lined with carborundum, kaolin, and water glass. At the Extension it is dried, fluxed, and smelted in oil-fired Steele-Harvey tilting furnaces which contain a No. 250 graphite crucible, while at the Tonopah mill the fine zinc- shaving precipitate is incompletely dried, mixed with crude borax which swells up through the mass, and then smelted in six coke-fired tilting furnaces. Crucibles last from 90 to 130 hr. and are turned once. Tonopah bullion will average 950 fine in silver and a trifle over 10 in gold and is sampled by being bored at opposite corners of top and bottom bars. The bullion is shipped by freight like any other merchandise.
The Montana Tonopah closed its 500-ton mill in 1923, and thereafter no operating mill in the district employed concentration.
The average gold extraction in this district was 94 per cent, and the average silver extraction 92 per cent.
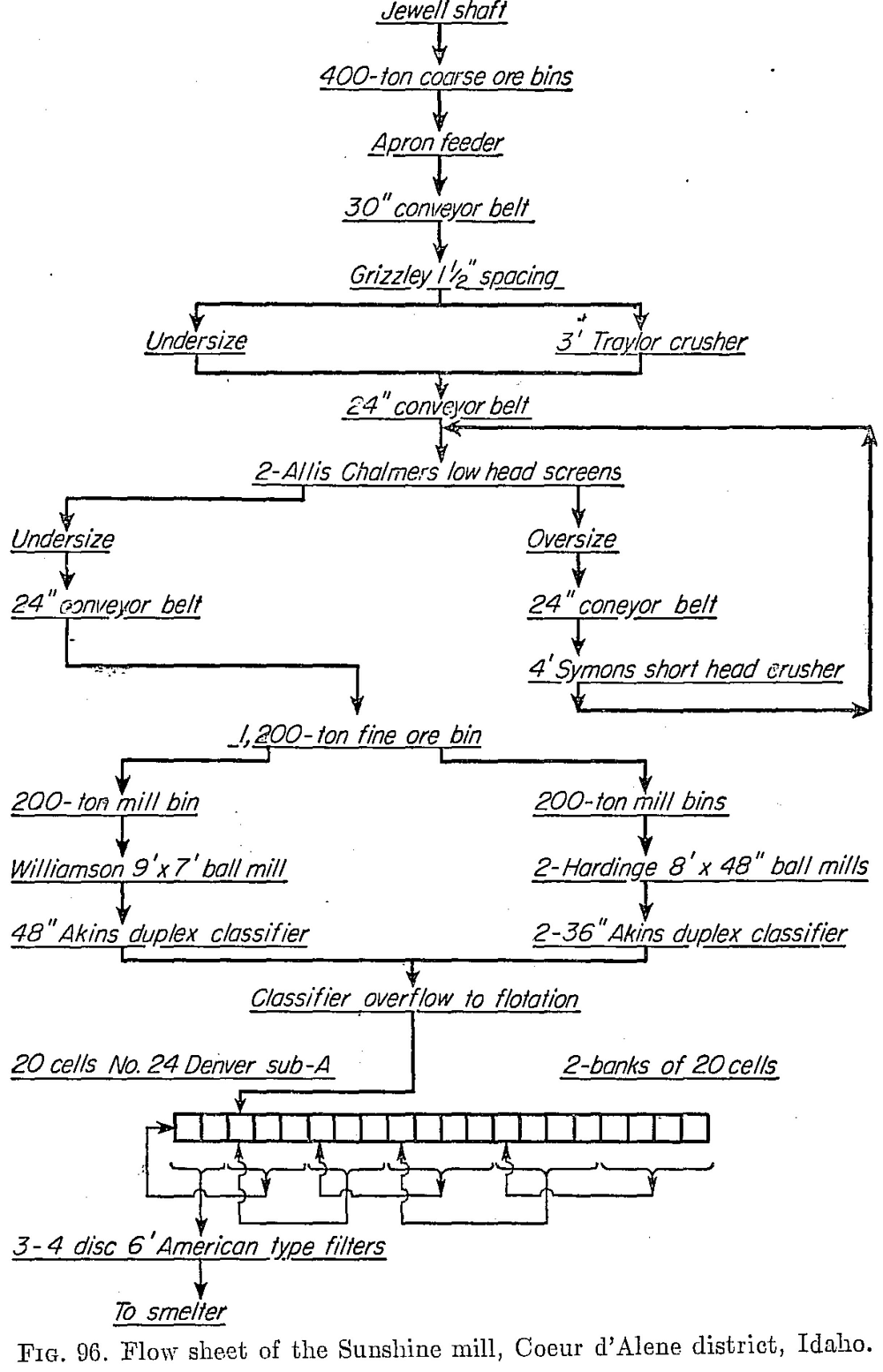
The Sunshine mill operated by the Sunshine Mining Company is situated 6 miles from Kellogg, Coeur d’Alene district, Idaho. The mine is the largest silver producer in the United States. In 1937 its output was 12,147,719 oz. silver and 2,784,289 lb. copper. In 1947, on a curtailed basis due to labor shortage, it produced 5,034,160 oz. silver, 1,249,555 lb. copper, and 5,881,796 lb. lead. The 1200-ton mill employs the straight flotation flow sheet shown in Fig. 96, the concentrate being shipped to a lead smelter. In 1947 the ore averaged 44.5 oz. silver (associated with galena and tetrahedrite, Cu8Sb2S7), 0.55 per cent copper, and 2.61 per cent lead. The recoveries were 98.53 per cent of the silver and 98.04 per cent of the lead. Milling costs were 95 cents per ton.
The flotation reagents used are 0.13 lb. per ton butyl xanthate and 0.75 lb. per ton frother. The frother is a mixture of 1 part Barrett No. 4 with 3 parts methyl amyl alcohol. Average concentrate analysis for 1947 is shown in Table 93.
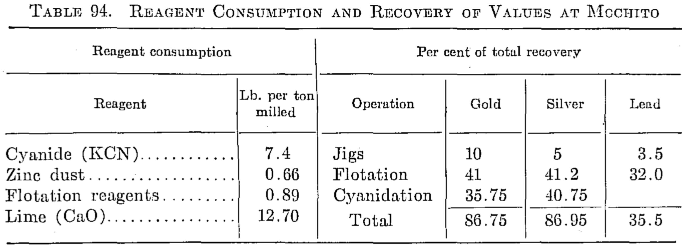
The New York and Honduras Rosario Mining Company operates two mills in Honduras, the Rosario and Mochito mills, and the El Dorado mill in El Salvador. The following information in regard to the latest practice at these mills was supplied to the author through the courtesy of the president of the company, W. A. Prendergast.
Rosario Mill (Type IIa). The mill treats 550 tons daily of an ore carrying 13.25 oz. per ton silver, 0.071 oz. per ton gold, 0.5 per cent zinc, 0.5 per cent lead, and 2.0 per cent manganese.
Primary crushing is carried out in two gyratory crushers making a 2-in. product, 350 tons of which is crushed in twenty 1800-lb. stamps and 200 tons in a 6- by 5-ft. Allis-Chalmers ball mill charged with 5-in. alloy-steel balls. This mill is in closed circuit with a 5- by 25-ft. 6-in. DSFXM Dorr classifier overflowing a 35-mesh product. The stamp milling is carried out in cyanide solution (3 lb. KCN per ton of solution). The product passing the ¾-in. battery screens is dewatered in two 6- by 20-ft. Dorr DSC classifiers, the overflow going to thickeners and the underflow to two 5- by 9-ft. ball mills in closed circuit with two 6- by 18-ft. DSC Dorr classifiers, also overflowing a 35-mesh product. A rationed ball charge of 70 per cent 3-in. and 30 per cent 4-in. moly-chrome alloy balls is used.
The minus 35-mesh product from both classifiers flows to an 8- by 6- by 20-ft. DSF classifier which is close-circuited with two 5- by 9-ft. ball mills using a 2-in. ball charge. The final pulp runs 26 per cent plus 150 mesh.
The total steel consumption for crushing and grinding is 1.86 lb. per ton of ore milled.
Four 35 by 10-ft. Dorr thickeners and one 35- by 15-ft. Dorr balanced-type tray thickener produce pulp underflows, by means of direct-connected 4- in. Dorrco diaphragm pumps, of 40 per cent solids. This thickened pulp is agitated for 83 hr. in batches in eighteen 15- by 45-ft. Pachuca tanks and in three 35- by 10-ft. Dorr mechanical agitators. The air pressure in the Pachucas is 35 lb. per sq. in., and about 95 cu. ft. per min. is used. The cyanide is added to the Pachucas to maintain a strength of 4.6 lb. KCN per ton of solution, and lime is held at 0.8 lb. per ton of solution. The cyanide consumption is 2.956 lb. KCN per ton of ore, and the lime consumption is 15.21 lb. of crude lime of 8.15 lb. CaO per ton of ore.
Filtration is done in three Merrill center washing slime presses with one hundred 3-in. by 4-ft. by 6-ft. frames, the plates covered with 8-oz. sail canvas, which has a life of 1100 charges or 79 days. The press cycle consists of charging with pulp for 10 min., a barren solution wash of 28 min., a water wash of 34 min. under 55-lb. pressure, and sluicing of the presses for 20 min. with water at 75-lb. pressure on the nozzles. For the washes at the presses 825 tons of barren solution and 1,025 tons of water are used; 2,425 tons of water are used for sluicing the presses. The dissolved- values loss in the tailings are 7 cents in silver and 3 cents in gold.
The precious metals are precipitated from the solution by means of zinc duct of which 0.4735 lb. per ton of ore milled or 0.03929 lb. per fine oz. of bullion is consumed. The pregnant solution averages about $2.88 per ton, and 2000 tons is precipitated per 24 hr. The effluent carries a trace of the metals.
Thirty-five per cent of the silver and 71 per cent of the gold are dissolved in the grinding circuit, 55 per cent of the silver and 24 per cent of the gold during agitation and 0.8 per cent of the silver and 0.7 per cent of the gold in the filters.
In 1948 the Mills-Crowe cyanide recovery process regenerated 174,066 lb. KCN from the barren solution. This enabled the carrying of a high cyanide strength in the agitators, a longer water wash on the Merrill filter presses, and a low mechanical loss in the tailings from the filters.
El Mochito Mill
This mill, which is located at Mochito, near Lake Yojoa, treats 100 tons per day of a high-grade silver ore carrying about 39 oz. silver per ton. It has a relatively high manganese content (4 per cent) mostly in the form of pyrolustite. The silver occurs with lead and zinc sulphides.
The ore is delivered from the mine to the mill, a distance of about a mile, by means of Diesel trucks. The ore is crushed to a 1½-in. product through a No. 3 gyratory crusher. Primary grinding is done in a 6 by 5 Allis-Chalmers ball mill in closed circuit with a Denver mineral jig and a 4-ft. by 18-ft. 4-in. Dorr DSFH classifier, and the overflow carries about 10 per cent plus 150 mesh in the pulp. The pulp from the secondary classifier is thickened in a Denver 38- by 10-ft. thickener, the underflow
going to eight Massco Fahrenwald flotation cells, five of which are used as roughers, two as cleaners, and one as recleaner. Pine oil, Aerofloat 31, reagents 404 and 301 are used. The pH is maintained at about 7.6. Flotation tailings are thickened in a 38- by 10-ft. thickener to about 40 per cent solids, the underflow being elevated by Oliver slurry pumps to two Denver disk filters, 6-ft. diameter and five leaves each.
The cake from the filters, which carries about 20 per cent moisture, is repulped in barren cyanide solution from the precipitation plant and is then aerated in an 8-ft. Denver agitator, where the lime emulsion is added. Cyanide is then added to the pulp as it flows to agitation. The pulp is agitated 63 hr. in six 12- by 36-ft. Pachuca tanks, the cyanide being- maintained at about 6 lb. KCN per ton of solution and the lime at 0.8 lb.
The pulp from the Pachucas then flows to four 38- by 10-ft. counter¬current washing thickeners. Two 8- by 10-ft. Oliver filters are now being installed for filtration of the pulp from washer 4 in order to lower the mechanical loss in cyanide, which is high because of the high cyanide strength required during agitation.
The precious metals are precipitated from 400 tons of solution daily by the Merrill-Crowe system using zinc dust.
Dorado Mill (Type IIa). This mill, located at San Isidro, El Salvador, treats 100 tons per day of an oxidized ore carrying 1.61 oz. per ton silver and 0.25 oz. per ton gold.
The ore is very hard, and about 3.25 lb. of balls is consumed per ton in grinding. Due also to the large quantity of wet clay present, it is necessary to wash the ore before crushing.
The washing is done in cyanide solution by means of a 4- by 19-ft. washing trommel with a 1-in. punched-plate screen. The undersize is dewatered in a Stearns-Roger dewatering drag 4 by 26 ft., the overflow of which, containing about 11 per cent of the tonnage, is pumped direct to the primary thickener. The trommel oversize is discharged onto a 24-in. picking belt where waste is hand-picked and the ore delivered to a No. 50 Kue-Ken crusher set to crush at 1½ in. The crusher product plus the dewatered drag sands are delivered to the fine ore bin at the grinding plant by means of a 16-in. by 280-ft., 15-deg. inclined conveyor.
Primary grinding is done by means of a 6- by 5-ft. Marcy ball mill in closed circuit with a 3- by 15-ft. Wemeo screw classifier. The secondary grinding is carried out in a 4- by 10-ft. ball mill charged with 1.5-in. balls and in closed circuit with a 6-ft. by 21-ft. 4-in. model F Dorr classifier, the overflow being all minus 150-mesh product and flowing directly to the primary thickener, which is 38 ft. 7 in. in diameter by 10 ft. deep. The underflow is maintained at 40 to 45 per cent solids and is agitated in three 21-ft. 6-in. by 16-ft. mechanical Wemco agitators with an air pressure of about 12 lb. on the air lift. These are in series and flow into four 38-ft. 7- in. by 10-ft. washing countercurrent thickeners, all underflows being maintained at 40 to 45 per cent solids.
Six hundred tons of pregnant solution is precipitated daily by means of the Merrill-Crowe system, the precipitates being collected in a bag unit.
Owing to the colloidal slimes present in the ore, it has been found necessary to add to the washing solution 0.015 lb. caustic starch and a lime emulsion before washing the ore in order to get flocculation of the slimes. About 10 lb. of lime is consumed in washing the ore.
PROCESSING MANGANESE SILVER ORES
Oxidized silver ores containing the higher oxides of manganese are generally refractory to metallurgical treatment. Manganese fouls mercury if amalgamation of the gold content is attempted. A refractory compound of manganese and silver is formed, probably a manganite, which is insoluble in cyanide solution and other common solvents for silver.
Caron Process
The Caron Process (U. S. Patent 1,232,216, Aug. 3, 1917), described by G. H. Clevenger and M. H. Caron in Bul. 226, U.S.B. of M., is based on the following principle: When oxidized ores containing a refractory compound of manganese and silver are heated in a reducing atmosphere, the higher manganese oxides are reduced to manganous oxides, and if cooled so as to prevent reoxidation, the refractory compound is rendered amenable to cyanidation. Refractory compounds of silver also can be so treated.
Manganese-silver ores occur generally in acid-eruptive rocks, chiefly rhyolite and dacite flows of later Tertiary age. Potassium-aluminum silicate is a vein material, and the vein quartz replaces the calcite. The manganese oxide is generally of secondary origin and is formed by atmospheric agencies. For the foregoing reason manganiferous ore from near the surface may be refractory but from depth may be amenable to treatment. “Wad,” a hydrous manganese manganate, is common in the zone of oxidation.
Various treatments of the raw ore have proved unsuitable—concentration (including flotation), magnetic separation, chloridizing, roasting, volatilization, sulphuric acid, and heating with organic matter. The Ag to Mn ratio persists in all sieve sizes from plus 20 to minus 200 mesh.
Laboratory tests were made in the United States and in Sumatra, followed by plant-scale runs in the latter country and a 50-ton plant at Pachuca, Mexico. Direct cyanidation of raw ore containing 2 to 10 per cent MnO2 gave 50 per cent extraction of the silver, but ore with 25 per cent MnO2 gave only 25 per cent extraction. The Caron process, on the other hand, extracted 92 per cent of the gold and 90 per cent of the silver. The pilot plant in Mexico successfully treated ore containing 2.8 to 13 per cent MnO2 and 12 to 20 oz. Ag. The Clevenger kiln (U. S. Patent 1,379,083, May 24, 1921) was fired with producer gas with the following analysis: CO, 15 per cent; CH4, 5.5 per cent; H2, 4.6 per cent; CO2, 6 per cent, the remainder being nitrogen.
The general conclusions as to the operation and efficacy of the Caron process are:
- Size of ore fed to the rotary kiln may be as coarse as 1 to 2 in.
- Producer gas of 150 B.t.u. or higher, made from any fuel, may be used.
- Temperature range is 500 to 700, best at 600°C.
- Calcine should be discharged into an inert atmosphere or directly into cyanide solution.
- Unaltered MnO: after calcining should be determined.
- Alkalinity control is important.
- Excess air should be used during grinding and agitation.
- Gold in some manganese-silver ores is amenable to direct cyanidation, and its extraction is not increased by reduction. Gold in other ores follows the silver and is more or less refractory and is benefited as much as silver by reduction.
- Silver extraction of 60 per cent from raw ore may be increased to from 88 to 96 per cent.
- Cyanide consumption is not affected by ,MnO2.
- Lime consumption may be 16 lb. per ton.
- A daily economic minimum-treatment plant for these ores is 200 tons, and the cost could approach $1 per ton, the equivalent of dead roasting and treating a low-sulphur ore.
Caron described the application of the process at Tambang Sawah, Sumatra, Dutch East Indies, a translation appearing in the M.J. (London) Feb. 19, 1927. The ore, mainly quartz, carried 18 per cent manganese dioxide, 30 oz. silver, and 8 dwt. gold. After being crushed to 1 in., it was heated 4 hr. in a rotary kiln. It remained 1½ hr. in the reducing section of the lain in producer gas at 600°C. The MnO2 was reduced to 2 per cent. The ore was then ground and cyanided, yielding 87 per cent of the silver and 97 per cent of the gold, as compared with 25 per cent by raw treatment. Chemical consumption was 2.2 lb. cyanide, 5.5 lb. lime, and 1.3 lb. zinc per ton ore.
A very complete bibliography covering the treatment of manganese-silver ores is given in Bul. 226, U.S.B. of M., 1925, by Clevenger and Caron.
McClusky Process. Manganese is found in varying percentages in the ores at Fresnillo, Mexico; although, fortunately, the average content is not enough to necessitate special treatment, ores from some parts of the mine contain sufficient manganese to affect seriously the extraction of the silver. To improve the extraction on this relatively small quantity of refractory ore, S. P. McClusky, formerly metallurgist with the Fresnillo company, developed a modified method of what has become known as the sulphur dioxide process for manganiferous silver ores. This is described by W. E. Crawford in Trans. 112, A.I.M.E., 1934, as follows:
Briefly, the method consists of:
- grinding of ore in water,
- subjecting the pulp to the action of sulphur dioxide gas to dissolve the manganese minerals,
- precipitating the dissolved manganese with a lime emulsion,
- aerating the pulp, and finally
- cyaniding in the usual manner. The ideas involved in the method are
(a) that part of the silver is in too close association with the manganese minerals which inhibit the action of cyanide solution on this silver:
(b) that, when these minerals are dissolved by SO2, the associated silver is liberated and thereby becomes accessible to the solvent action of the cyanide solution.
Moreover, if the dissolved manganese is then precipitated by lime emulsion and oxidized to the manganic state by aeration, it no longer affects extraction, although it still remains in the pulp.
The content of silver in the ore and the gain in extraction by sulphur dioxide treatment, plus the cost of the process, are the criteria by which the applicability of the process to manganiferous silver ores may be judged. At Fresnillo argentite is the predominant silver mineral. It is associated with pyrite and manganese minerals.
In practice, the ore is ground in a weak, “spent” solution of 0.008 per cent KCN so that 30 or 35 per cent passes through a 200-mesh sieve and 1 per cent is coarser than 10 mesh. The pulp then flows to a 4-in. Wilfley centrifugal pump, which delivers the pulp to the top of the first and second of the sulphur dioxide treatment towers, which are sealed, airtight chambers, three in number. These towers are of wood, 3 by 3 ft. in cross section and 17 ft. high, with wooden baffles lined with white- iron plates. The purpose of the baffle plates is to disperse the pulp as it falls through the tower, so that it may come into intimate contact with the ascending current of SO2 gas. The flow of the pulp and SO2 gas is countercurrent, the pulp is constantly enriched in acidity and the gas mixture progressively depleted of SO2 from unit to unit. The final result is that the gas exhausting to the atmosphere contains slightly more than 1 per cent SO2 indicating a total absorption of 85 per cent of the available SO2. The gas used in this process and in the cyanide-regeneration plant at Fresnillo is produced by roasting the pyrite from flotation in seven-hearth Herreshoff furnaces.
From the SO2 absorption towers the pulp passes through five conditioner tanks arranged in series. An emulsion of lime is added to the fourth tank for the purpose of precipitating the dissolved manganous and ferrous compounds, as manganous and ferrous hydrates. The low-pressure air in this and the fifth tank assists in oxidizing the manganous and ferrous compounds to manganic and ferric compounds. After passing through the last conditioner tank the pulp is returned to the mill for regrinding in a 6- by 14-ft. Traylor ball mill in closed circuit with a Dorr bowl duplex classifier. The overflow of this classifier, which averages 60 per cent minus 200-mesh material, joins the feed of the plant treating the regular silver ore.
The gain in extraction accomplished by the sulphur dioxide treatment varies considerably with different ores, but it appears to be in direct proportion to the amount of manganese dissolved by the gas, approximately 7 grams silver for every 0.1 per cent dissolved manganese. An increased recovery by this treatment, of 25 grams silver per ton, represented a substantial economic advantage when silver was quoted at around 30 cents (United States currency) per ounce.
The laboratory pilot test, carried out daily in conjunction with the plant treatment, often showed as much as 35 grams additional recovery of silver. Mixing of the SO2 treated slimes with the general mill slimes made it difficult to check the actual additional recovery in the plant.
An interesting point is noted in connection with tests for the oxygen content of solution in the pulp leaving the final treatment tank of this unit. This solution is entirely devoid of free oxygen; moreover, it required several hours of vigorous agitation with air to satisfy the oxygen-consuming requirement and to render it susceptible to the absorption of free oxygen. In view of this, it is quite possible that a separate cyanide circuit for these treated slimes would be a distinct advantage, especially if it were so designed that several hours of agitation and aeration could be given prior to the addition of cyanide.
Cyanidation and concentration of gold and silver ores
Types of Silver Ores with Possible Extraction Methods
- Silver sulfide ores (amenable to flotation-smelting recovery techniques).
- Oxidized, clean silver ores (amenable to cyanidation recovery).
- Oxidized silver ores resistant to cyanidation recovery. Frequently these ores contain manganese.
- Complex silver sulfide ores such as tennantite, tetrahedrite, and other combinations of silver with arsenic and antimony. (Suitable for flotation).
- Argentiferous galena ores. (Flotation).
- Argento-jarosite ores. (Difficult at best).
- Silver ores with sulfide manganese. (Suitable for flotation).
Possible Extraction Methods
There are several approaches to the problem of silver recovery from manganiferous ores. These are listed with the knowledge that particular ores may be economically amenable to some unmentioned processes and that not all attempted processes are known to the author.
- SO2 leach, followed by thorough washing, liming to a suitable pH, and standard cyanidation.
- Chloride oxidation roasting followed by cyanidation or amalgamation.
- The venerable Patio Process – a combination of treatment with copper sulfate plus sodium chloride, followed by amalgamation for recovery of the silver.
- The Washoe pan amalgamation process.
(All processes using mercury today are environmentally difficult). - The Kerley thiosulfate leach process. This process is said to be effective for problem ores containing high percentages of manganese, copper, arsenic, tellurium, etc., or combinations of the above. The process is being offered by ThioTech of Sahuarita, Arizona.
- Direct smelter recovery of high grade ore or concentrates.
- A chloridizing reduction roast (Segregation Process) followed by flotation. This could be particularly useful in a copper-silver-manganese ore such as that from the Berenguela Mine of the Lampa Mining Co. near Puno, Peru. It could also be used on a combination of a refractory Ag ore mixed with an oxide copper ore.
- Flotation where suitable (sulfides).
- Brine leach (as the tetrachloro-complex).
Applicable Chemistry of Silver Extraction
Chloride Roasting Followed by Cyanidation:
Ag2S + 2NaCl + 2O2 → 2AgCl + Na2SO4
AgCl + 2NaCN → NaAg(CN)2 + NaCl
Chloridizing Leach:
AgCl + 3 NaCl → Na AgCl4 (soluble in excess brine)
Chemistry of Manganese Removal From Tie-Up With Silver:
Also MnO2 + SO2 → MnSO4 (somewhat pH dependent)
MnO2 + 2SO2 + ½O2 + H2O → MnSO4 + H2SO4
(The generated H2SO4 helps to dissolve divalent Mn).
MnO + H2SO4 → MnSO4 + H2O
(also soluble in H2SO4 is the iron portion of jarosite).
Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
Product Recovery Methods
In all the above methods where cyanidation is used, product recovery is frequently accomplished by zinc dust precipitation with subsequent fire refining. In cases of gold-bearing ores, concentration of values from low-grade cyanide solutions by adsorption on activated charcoal with subsequent electrowinning of a dore bullion is a useful method but in the case of silver ores running several or more ounces per ton, the sheer bulk of the necessary carbon points to the direct zinc dust precipitation method as preferable. An alternate precipitation method involves the use of NaHS or Na2S as suggested by Reno USBM to precipitate a silver sulfide product. Use of this method has been held back by poor settling and filtration rates for the silver sulfide precipitate and the insidious toxicity of possible hydrogen sulfide gas, to say nothing of hydrogen cyanide.
The recovery methods employing sulfur dioxide to dissolve the manganese offer the possibility of a manganese recovery step. This may be economic in those ores containing substantial manganese values. Parenthetically, it is felt that even very low values of manganese may be sufficient to render the silver difficult to dissolve with cyanide if the manganese and silver are syngenetic.

