Some of the fundamental principles of this concentration ‘upside down,’ as it may be termed, being such a new method, have been overlooked. There has been such a mad scramble to get results in advance of the ‘other fellow,’ and to penetrate the cloud of secrecy enforced by patent litigation, that there has been little time to answer the question as to why the heavier mineral floats and the lighter gangue sinks. In this buzzing cloud of secrecy the student can distinguish such phrases as ‘froth flotation,’ ‘surface tension,’ ‘oil- films,’ ‘DeBavay float,’ ‘liquid skins,’ etc., all of which tend to confuse rather than answer the main question. A few articles in the magazines have given various data, phenomena, causes, and effects, but no definite theory explaining these has been clearly stated.
The action of any flotation machine in successful operation seems quite simple, the mineral floating in preference to the gangue, giving rise to the phrase ‘selective flotation.’ All that is necessary for the one type of machines is to place the mineral particles gently on the surface of a liquid so that they will not sink or, in the other type of machines, attach to them something of a lighter specific gravity than the liquid so that they will rise bodily to the surface. On the face of it, this is quite simple. Apparently the simplest of all is to attach ‘life-preservers’ or something buoyant to the mineral particles.
Herodotus describes how “the virgins drew up gold by means of feathers daubed in pitch.” Therefore this or an oil, for instance, can be employed to float mineral. The Elmore patents for this flotation, due to the buoyant property of oil, are still in effect. Owing to the large quantity of oil necessary, as well as other things that make this method of no commercial value at present, the only ‘why’ to be considered in this class of flotation is the selective action, which will be discussed later.
The simplest and cheapest ‘life-preserver’ is undoubtedly the pneumatic one, which is beyond the time of Herodotus or perhaps even history itself, since eggs and cream were surely frothed before the stylus was known. Any little girl who has helped her mother in the kitchen can tell how any foreign substance, such as a piece of egg-shell for instance, is buoyed up and brought to the surface by these bubbles. These are bubbles of air, and what could be cheaper for the manufacture of these simple pneumatic ‘life-preservers’? It is true that Delprat, with his process, uses carbon dioxide, but this is simply a case of using a by-product that would otherwise go to waste.
Therefore the whole sum and substance of this apparently complex problem of ore concentration by flotation, now surrounded by a cloud of secrecy, consists in either attaching mineral particles to gas bubbles, preferably air, or attaching air bubbles to mineral particles. It amounts to the same thing whether the bubble be attached to the solid or the solid attached to the bubble. In the one case, ‘surface tension’ type of machine is used for the concentration, while in the other a ‘froth’ type of machine is used. These will be taken up later.
Therefore the two prime requisites to flotation are:
- Attachment of bubbles to solids;
- Creation of selective action of bubbles for metallic particles instead of for the gangue particles.
Since the all-important requisite is the attachment of gas bubbles to solids, it is logical to investigate this phenomenon first with the simplest material at hand—air and water.
Far back in primeval time the progenitors of the fishes made air bubbles that came to the surface of the water and yet we, a few years ago, knew but little more concerning air bubbles in water. There was no reason for these aquatic things knowing the ways that air can exist in water but there was no excuse for our building Pachuca tanks, blowing air in at the bottom and then writing a beautiful chemical equation to show that these air bubbles were necessary for dissolving gold. Visible bubbles of air, which have been blown into the water, can in no possible way sustain the life of a fish. Neither can they aid one iota in dissolving gold in a cyanide solution. Nor can visible bubbles of air, introduced into a liquid in this way, be attached to solids to aid in flotation. Available air or oxygen for things of this nature must be air actually in solution. A fish may be breathing freely at the bottom of an aquarium. If the water be warmed so as to expel the air, the fish will rise to the surface and try to jump out. Only nascent hydrogen can unite with arsenic in the Marsh test. In the same way, only nascent oxygen from the dissolved air can act as shown in the chemical equation:
2 Au+4 K (CN) +H2O+O=2 KAu (CN)2+2KOH.
when gold is dissolved. Only nascent gas can be attached to mineral in the flotation process. This fact is quickly demonstrated by introducing a small jet of air into the bottom of a flask, filled with water, where rests some pulverized ore, the metallic particles of which are in a perfect float condition. Why will the mineral particles, which almost float of their own accord, refuse to attach themselves or be attached to the small bubbles of air? To prove that these same metallic particles can be floated by bubbles of air, it is only necessary to remove the jet and place the flask on a hot plate when they will immediately collect air driven out of solution by the heat and rise to the surface. Some one may here remark, that the rise of temperature of the solution causes enough expansion of the air bubbles already attached to the metallic particles to produce flotation. Anyone familiar with the law of Henry will know this is not the case on noting the greatly increased size of the bubbles. Why, then, cannot air bubbles be attached to mineral particles in the place of nascent or dissolved air?
All great facts, when thoroughly understood, are demonstrable by simple experiments with material at hand. Sometimes when the young man at the soda fountain absent-mindedly forgets to stir your cherry phosphate and sets before you the straws, demonstrate the above fact to your satisfaction while the nascent bubbles of CO2 form and rise to the surface of the liquid. Crush the straw slightly to reduce the size so that only a minimum of air can be forced through. With this straw, blow air into the colored syrup in the bottom of the glass so as to form a few small bubbles that can be watched closely. These bubbles that come to the surface are colored. Why? The air itself is not colored. Therefore, since the only part of the liquid that is colored, is in the bottom of the glass, the air must be enveloped in the same identical portion of liquid throughout its passage from the bottom to the top of the glass. In other words, the air bubble, on being introduced into the liquid, is immediately surrounded and inclosed by a film of liquid, which remains with that air bubble throughout its passage just as if it were a part of the bubble. Here is a concrete example of surface tension, a force that can be measured, as explained in any text-book of physics.
This phenomenon is worthy of investigation. The bubble rises to the surface of the liquid by reason of the force of gravity. That is, the force of gravity is greater than adhesion of the molecules of the air for the molecules of the liquid; otherwise the air would remain in the liquid. The molecules of the liquid move freely among themselves according to the definition of a liquid. In other words, the force of cohesion of any single molecule within the liquid is equalized by the cohesive force of other molecules of the liquid. An extraneous force would be required to separate them. An air bubble, for instance, introduced into the liquid, unbalances this cohesive force. It is self-evident that this force of a molecule must act equally in all directions from that molecule. Therefore molecules of the liquid adjacent to the air bubble have their force of cohesion on the one side satisfied by that of adjacent molecules of the liquid; while, on the side of the air bubble, there are no molecules of the liquid to equalize this force. Being statical, this force must be equalized by that of adjacent like molecules in a transverse direction. Since a force of cohesion was already in existence between these adjacent molecules this force is thereby multiplied so that there then exists a greater cohesive force between the molecules immediately surrounding the air bubble than that existing between the molecules in the interior of the liquid. This force is ‘surface tension;’ it is so great that these molecules of the liquid surrounding the air bubble are firmly held together and torn loose from adjacent molecules of the liquid as the bubble rises to the surface. That is to say surface tension causes the molecules of the liquid to form a film around the bubble and remain with it to the exclusion of like molecules during the time the bubble remains in the liquid. To all intents and purposes, this film is seen to be the same as if it were a membrane of some solid. The air in these bubbles can no more come in contact with the liquid through which it is passing than it could were it inside a toy balloon, for instance. The bubble may be said to be enclosed in a ‘liquid skin’ Therefore to attach this bubble to any substance, this liquid skin must first be penetrated or broken. As seen from above, this requires some force.
As shown above, the force of chemical affinity is not sufficient to overcome this surface tension. So then, it could hardly be expected that a mere adhesive force would be greater than this surface tension. Therefore, to attach gas to solids in a liquid, it is first necessary to dissolve the gas in the liquid and then expel it in a nascent state.
There are at present only three known ways of forcing a gas mechanically into solution so that it actually occupies the interstitial spaces of the liquid molecules:
- beating it in with stirrers or paddles, as is described, for instance, in the patent papers of the Minerals Separation Co., where propellers or centrifugal pumps are used;
- dividing it into such minute portions that, by capillary force, it is actually taken into solution, as is done in a Callow cell; and
- introducing it as a surface film surrounding a jet of fluid by means of surface tension, as is done by a method under process of patent.
There are also three methods of expelling dissolved gas from a liquid that are of vital interest to the matter in hand;
- Super-saturation, so that the excess gas comes out of its own accord;
- heating, which expels some of the gas by increasing its volume; and
- reduction of pressure. The present Elmore machines work the pulp in a vacuum, taking advantage of the fact that “at constant temperature, the gas dissolved in a given volume of liquid varies directly as the pressure”—Henry’s law.
Since it is easier to work in the open air than in a vacuum, flotation machines using the principle mentioned, of forcing more air into solution than the liquid can hold, are preferable. The second method mentioned, of expelling dissolved gas by heat, aids the super-saturation type of machine in two ways:
- nascent gas is expelled from the liquid to be readily attached to solids for flotation; and
- dissolved gas is expelled from the solids so that gas bubbles may be easily attached to them. Here lies the whole secret of flotation.
No solid can be floated unless it contains some dissolved gas. Why ? For the reason, explained above, that the enveloping ‘liquid skin’ cannot be penetrated or broken. It was shown above that a gas bubble is surrounded by a film of liquid. A solid in a liquid is, in the same way, surrounded by a film of the liquid, for the same reason. Therefore, in a liquid, the molecules composing the film around a gas bubble would have no more attraction for those composing the film surrounding the solid than they would have for any molecules in the liquid itself. Hence the bubble would not attach itself to the solid. It is seen then that flotation has for a foundation a subject of which practically nothing is known—occlusion of gases.
It is self-evident that the same cause which tends to super¬saturate a liquid with gas will also have the same tendency to super-saturate a solid contained therein. And also the same cause that tends to dispel from solution the dissolved gas will also tend to dispel the gas from a solid in this same liquid. Therefore a solid in a liquid becomes a nucleus for the formation of bubbles. This is easily demonstrated by the formation of vapor bubbles when water is boiled.
The surcharging of a liquid with a gas tends to surcharge any solid in this liquid—on account of diffusion. The adhesion of the gas for the solid, therefore, will tend to condense the gas on the surface of the solid. Sufficient condensation will collect enough molecules of the gas to form a bubble on the surface of the solid.
The same effect, due to diffusion of gas in the opposite direction, will be produced by causing the gas to be expelled from either the liquid or a solid contained in this liquid. An example of this is the dumping of a cold ore into the hot solution of a flotation plant. Bubbles immediately tend to form on the ore particles, by reason of cohesive and adhesive forces, and have the tendency to be enlarged by the gas in solution in the liquid.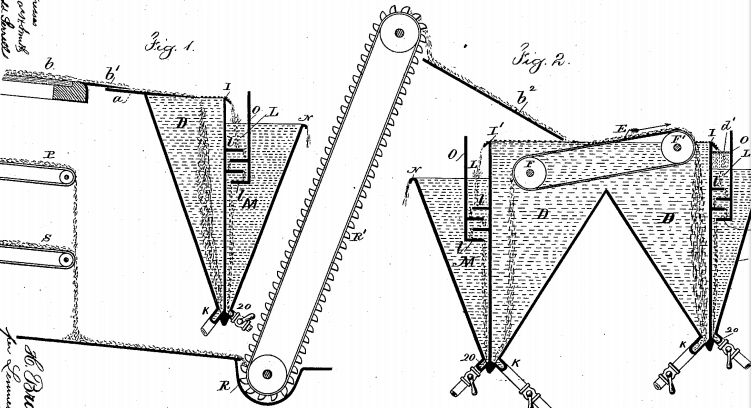
It is natural, therefore, to suppose that solids with high occlusive power for gases have a greater tendency to float. Here, then, is a cause of selective flotation. Hezekiah Bradford’s patent No. 345,951 is the first to recognize this. Speaking of metallic particles, he states: “These floating particles appear to possess some peculiar qualities which repel water from their surfaces, especially when such particles are exposed, even momentarily, to atmospheric air.” Later this phenomenon caused trouble to, instead of benefiting, Hebron, who says in his patent No. 474,829, an interest in which is assigned to Carrie J. Everson: “I expel from such mineral and metal particles—the air and other gases—by producing as far as practical a vacuum—or, and preferably, by applying heat to the ore, thereby obtaining the desired expulsion of air and other gases.”
Why then do minerals (here in these patent papers meaning solids containing metal), and especially sulphide minerals, occlude gases more readily than other solids? It is only necessary to look into the subject of ore deposition for the answer. Primary sulphide ores are changed near the surface to sulphates, carbonates, oxides, etc.; in other words, chemical affinity assists sulphides in absorbing oxygen or carbon dioxide. Hebron and others discovered, by the aid of the microscope, that most mineral particles to be saved by concentration have larger pores and surfaces of larger extent than equal sized ‘gangue’ particles. This gives a greater chance for gas occlusion, which is another cause of selective flotation.
There is practically no adhesive force existing between oil or fatty substances and water. As a general rule, an oil is but slightly soluble in water or water in oil. Therefore water will not adhere to a surface wetted with oil or oil will not adhere to a surface wetted with water. Also an oil, due to its property of capillary attraction, has that power of entering solids. Therefore, owing to larger surfaces and pores, most metals and sulphides are capable of absorbing oil so that sufficient oil can be attached for agglomeration and flotation. This selective flotation, as mentioned above, is not now worth considering, because so large a quantity of oil is necessary.
Mickle’s experiments showed that none of the minerals tried hot, cold, or with reduced pressure floated on oil under any of the conditions where floating would take place on water. This was to be expected, since the specific gravity of oil is less.
What then is the potent factor for selective flotation? It is the ability to vary the “angle of hysteresis.” It has been seen from the above that solids occlude gas which can be expelled from them. If this gas be expelled from them when they are in a liquid at a time when gas is expelled from the liquid, they become the nuclei for the formation of gas bubbles which will float them under certain conditions. Now, therefore, if it be possible in an ore mixture to drive out a considerable portion of the gas from all the particles, there will be insufficient remaining in the ‘gangue’ to float it while the mineral containing more gas will float to the surface. It has been found, for instance, that sulphuric acid in very small quantity added to water will decrease the angle of hysteresis to that point where quartz and similar ‘gangue’ will sink, while that of the metallic particles remains practically unchanged.
Since an acid in very minute quantity will produce this effect, it is not due to rise in temperature or reduction in pressure, which would drive out the occluded gas. This must be caused then by no ordinary phenomenon. The only way that an acid can act in this manner is in the capacity of an electrolyte, especially when diluted to its dissociation point. That is, complete ionization exists. Yet with this extreme dilution, gas is expelled from a solid contained therein. In other words, equilibrium does not exist. Why? It is on account of these ions of the electrolyte which cause this displacement of equilibrium between the solution and the gas dissolved in the solids within this solution. This then resolves itself into a simple case of osmotic pressure. The surface of the solid is the septum. The ions of the electrolyte enter the solid while those of the gas leave. Since the carrying solution is saturated with gas already, bubbles form; and this action continues until the eutectic point is reached. So far an acid (sulphuric on account of its cheapness) has been used as the electrolyte, because it produces such a great change in the angle of hysteresis.
In the future, as more is learned concerning flotation, the finer and more delicate manipulation will be better understood, permitting an alkaline electrolyte to be commonly used. This will allow of the selective action for mineral particles other than sulphides so that, for instance, cerussite or malachite can be separated readily from gypsum, quartz, etc. This is not to be confused with Horwood’s “differential” or “preferential” process, whereby the surfaces of some sulphide minerals are oxidized by roasting to prevent them floating with another sulphide in a mixed sulphide ore.
While, as stated above, the fundamental requisites are the manufacture of ‘life-preservers’ and the attachment of these to the mineral particles, it is still necessary to rescue these particles. Bubbles, on coming to the surface of a liquid, burst if not protected, and the attached mineral particle sinks. Why do they burst?
- Relief of pressure, so that the contained gas expanding exerts more pressure on the liquid film,
- adhesive force of contained gas for the atmosphere, or
- evaporation of the film causes this bursting. The greater the super-saturation, the greater the interior gas-pressure of the bubbles, so that they in reality explode. This is the case with bubbles in a glass of soda-water, for instance. How can this be prevented? The small boy mil prevent it by coating the bubbles with soap—that is, by toughening the liquid film. This then is the secret of “the froth-forming material” so frequently mentioned in the various patent papers of the Minerals Separation company. Why is an oil the most useful substance with which to do this?
It has been shown above that metallic particles are readily coated with oil. Therefore, an oil may not only toughen the bubbles but a cohesive force is exerted on the oil-coated metallic particles. Besides an envelope to hold the gas, an aeronaut uses a net to strengthen his balloon, so that when the pressure is relieved by the higher atmosphere it will not burst. This same effect is obtained in froth-flotation. In the same way that particles form around drops of water on a dusty floor and prevent the globule from breaking, small particles form a network around the large bubbles. This is due not only to the force of cohesion of the oil on one particle for that on another, but the force of cohesion existing between the particles themselves. Thus a froth is formed of bubbles that do not readily break.
It is a well-known fact that water has the greatest surface tension of all liquids under ordinary conditions, except mercury. It is there¬fore a safe assumption that dilution with another liquid will decrease the surface tension. The tendency to float is decreased. With reduced surface tension bubbles burst more readily. From this it is easily seen that surface tension is decreased exceedingly by the use of a volatile liquid. Alcohol evaporating from a substance held near a bubble will diffuse sufficiently to readily dilute the surface film and quickly burst it. Mineral particles floated when, for instance, amygdaloidal or globulous eucalyptus oil is used will dance on the surface of the liquid, being apparently attracted and repelled until evaporation has progressed sufficiently to equalize the surface tension not only of the liquid but of the bubbles as well.
Water then is the natural and universal medium for all flotation machines and air the necessary adjunct. The air may be in the pores of the mineral particles and as films around them, so that they are not easily wetted, in which case the machine may take some such form as a Macquisten tube or Henry E. Wood type—a purely surface- tension effect into which enters nothing but water and air. The meniscus of the water buoys up the metallic particles surrounded with an air film that prevents them being wetted. The force of gravity is less than that of surface tension, so the particles float. If the particles be surrounded by a water-film, the cohesion of the molecules of this film for those of the body of water neutralizes the surface tension, and gravity sinks the particles. Or again, minute bubbles may be attached to metallic particles that necessarily contain occluded gas. A thin film of oil may enclose or contain the particles and their attached bubbles. With sufficient displacement the particles will rise to the surface and form what may be termed a DeBavay float. Or, lastly, the bubbles may be large and have the mineral particles attached to them, as well as being attached to each other. This is the so-called froth flotation.
