Table of Contents
Borax a sodium borate and the principal sodium salt of boric acid, has been surrounded with romance and with a certain amount of mystery. Its early history is not entirely known but it has been contended that Marco Polo in his travels in the Orient introduced this material to the West during the Middle Ages. Without doubt its first source was from Central or Eastern Asia and perhaps from Tibet. The well-known fluxing properties of borax probably introduced its earliest use, that of a soldering flux in the manufacture of jewelry. This use, as well as the remote location of most borate deposits, added greatly to the romance associated with the thought of borax.
Composition
The common element in all borates is boron but this element has never been discovered as such in any deposit, because of its great affinity for oxygen. Incidentally, this makes its manufacture extremely difficult and expensive. Elemental boron undoubtedly has many properties that make it quite valuable but the extreme cost involved in producing it from its oxidized form prevents its general use or manufacture.
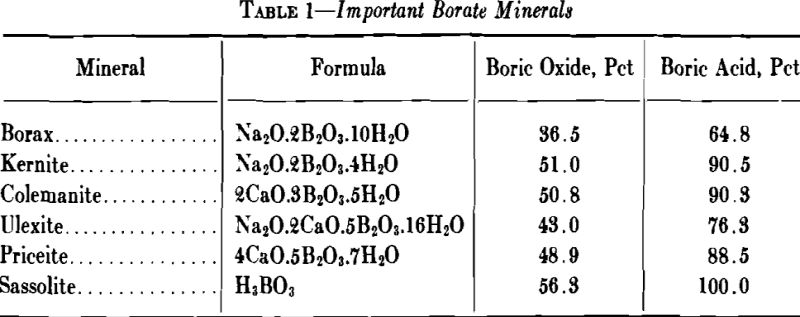
Many minerals contain boron; more than 50 have been listed and, excepting nine, they all contain water of crystallization or composition. Borates can be defined as minerals containing an oxide of boron, and usually water of crystallization, and include boric acid as well as metallic salts of boric acid.
Borax is the most important borate mineral both as a raw material and as a refined chemical. Chemically speaking, it is the decahydrate of disodium tetraborate (Na2B4O7.10H2O), and, further, can be considered as the acid salt of monosodium metaborate. However, the term “acid salt” might imply that borax gives an acid reaction in solution, which is not true, since solutions of borax are decidedly alkaline, giving a pH of 9.2.
Properties
Borax (tincal) in deposition is found as individual crystals, as aggregates of poorly developed crystals, and as compact glassy masses showing no crystal faces. When pure, borax is clear and colorless, but much of it is dull, earthy white. This earthy color is due to the partial dehydration to the lower hydrate tincalconite and, owing to enclosed mud, may even be gray or dark opaque. The crystals are monoclinic and have several cleavages but the natural mineral seldom shows cleavage faces when broken. Its hardness is 2 to 2.5 and its specific gravity is 1.72. Borax is soluble in water; although its solubility as well as its rate of solution is low at cold temperatures, it increases rapidly in hot water, and borax is completely soluble (melted) in its own water of crystallization at 77°C. When heated above this temperature a crystal of borax will lose its water of crystallization and finally becomes anhydrous at 740°C. On cooling it becomes a glass (borax glass) or, under special conditions, crystalline.
Distribution of Deposits
Of the six present commercially important borate minerals, four occur in California and Nevada in relatively large deposits; that is, borax, kernite, colemanite, and ulexite.
Although California probably is the present largest source of borates, large deposits occur in other countries. Ulexite is found in South America in Peru, Chile, Bolivia, and Argentina. Priceite (pandermite) occurs in commercial deposits in Turkey and is being mined today. Sassolite in solution occurs in the suffoni of Italy and supplies some of the boric acid needed in Europe and elsewhere. Stassfurtite occurs in Germany; crude tincal is found in Tibet, and ulexite has been found recently in Iran. As far as is known, all of these deposits are being commercially operated today.
Theories of Origin
The sodium borate of the Kramer region can generally be described as two layers of different sodium borates interspersed with shale. The lower layer is made of crystals of kernite interbedded with shale and the upper layer consists of borax and interbedded shale. A distinctive feature of the deposit is the total lack of water-soluble salts other than sodium borate. Any theory regarding the genesis of the deposit must account for this total lack of water-soluble salts as well as the formation of two sodium borates—kernite and borax (tincal).
Kernite is sodium borate containing four molecules of water; borax contains ten; furthermore, it is known through phase rule and equilibrium work that under normal pressure kernite can be crystallized only from a water solution when the temperature is above 60°C, and at concentrations that would almost certainly mean borax without any water other than its water of crystallization. Therefore, any theory regarding the origin would have to explain this high concentration of borate solution, high temperature necessary and also that other soluble salts at similar concentrations must have been removed without deposition. In view of all this, it is very difficult to understand the formation of the deposit through the flowing and evaporation of surface waters and the deposition therefrom of kernite.
Production
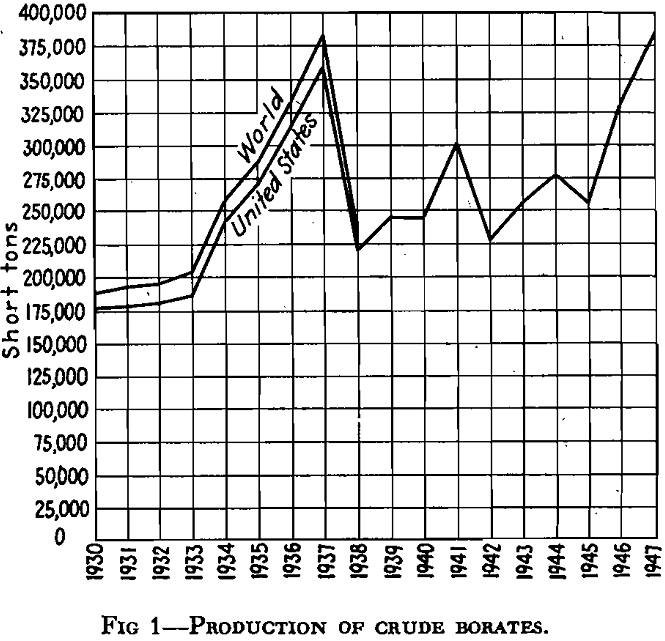
All figures showing production of borates can be seriously questioned as to accuracy and should be treated rather as rough approximations. As far as world production is concerned, this is obvious, since certain countries do not release such figures for publication; as far as domestic production is concerned, the sources are generally from government and slate publications which may be in terms of ore, in terms of borax, in terms of boric oxide, or even in terms of boric acid.
Mining Methods and Preparation for Market
At the present time practically the only borates being mined in the United States are in the Kramer district. These deposits are fairly thick and their location varies from 300 to 1000 ft below the surface, the average being about 400 ft. The mining method is that of shrinkage stoping, tramming, and hoisting through vertical shafts to the surface, where the ore is crushed to size suitable for concentration or refining into commercial borax.
Two methods are used for recovering the borax from the brine. The first method is that used by the American Potash and Chemical Corporation, wherein the brine is evaporated in triple-effect vacuum pans to remove, in addition to water, salt, sodium carbonate and sodium sulphate. From the resulting concentrated brine, potash is removed by rapid cooling. Finally, borax is crystallized from the solution and refined to commercial borax, which may be sold as such or made anhydrous by fusing in a furnace. Some of the borax is converted into boric acid through the addition of sulphuric acid to a borax solution.
The second method of producing borax from the brine is that used by West End Chemical Co., which consists of the removal of the sodium carbonate portion of the brine through carbonating with carbon dioxide produced from the calcining of limestone. Sodium bicarbonate so formed is removed from the brine and then, by cooling, borax is crystallized out, forming a crude borax. This borax is further refined by dissolving in water and recrystallizing. Pure borax is sold as such or is converted into anhydrous borax in a fusing furnace.
Uses
Borax is widely used for many purposes. Its easy solubility in water to a mildly alkaline and antiseptic solution, its low melting point and excellent fluxing properties make it one of the most useful of salts. Best known, perhaps, as a household commodity, it is of more importance in manufacturing, and many articles of everyday life require borax in their manufacture.
An excellent cleansing agent, it is widely used in industry and in the home, for washing, cleansing and laundering, either directly or as a constituent of soaps and soap powders.
Borax and boric acid find many applications in medicine and pharmacy, as in disinfectants, mouth washes, tooth powders, cosmetics, lotions, ointments, deodorants and medicated lint and gauze.