Titanium Electrorefining
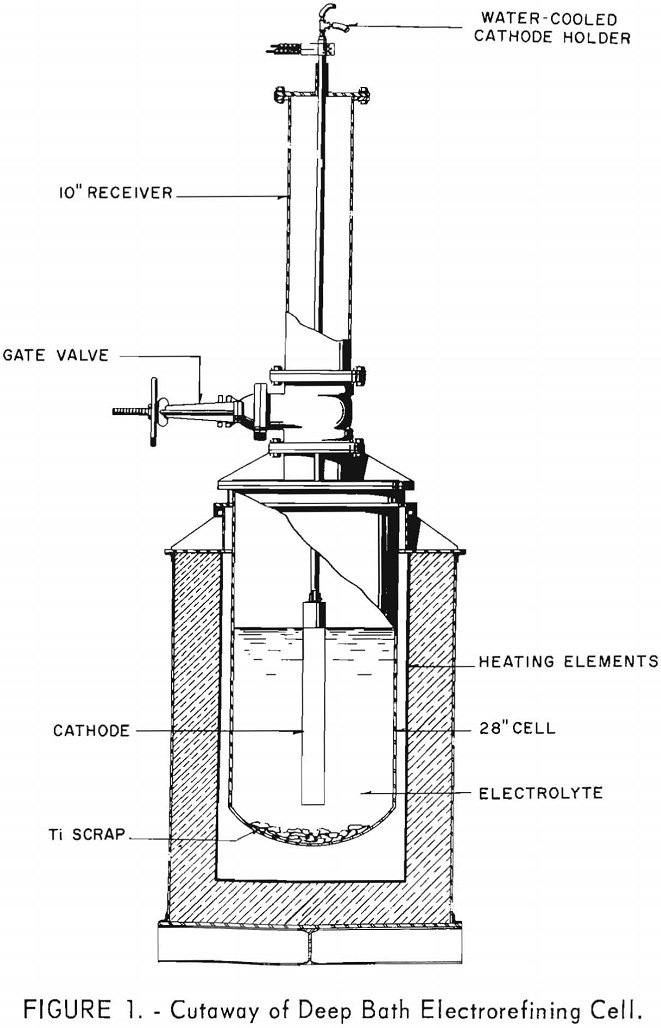
The development of a fused-salt Titanium Electrorefining technique to recover hyperpurity titanium from offgrade sponge and mill scrap, under investigation by the Federal Bureau of Mines Electrometallurgical Experimental Station at Boulder City, Nev., for several years has been described in many publications. This report describes the investigations to obtain greater capacity by increasing the depth […]
Electrorefining Titanium Electrowinning
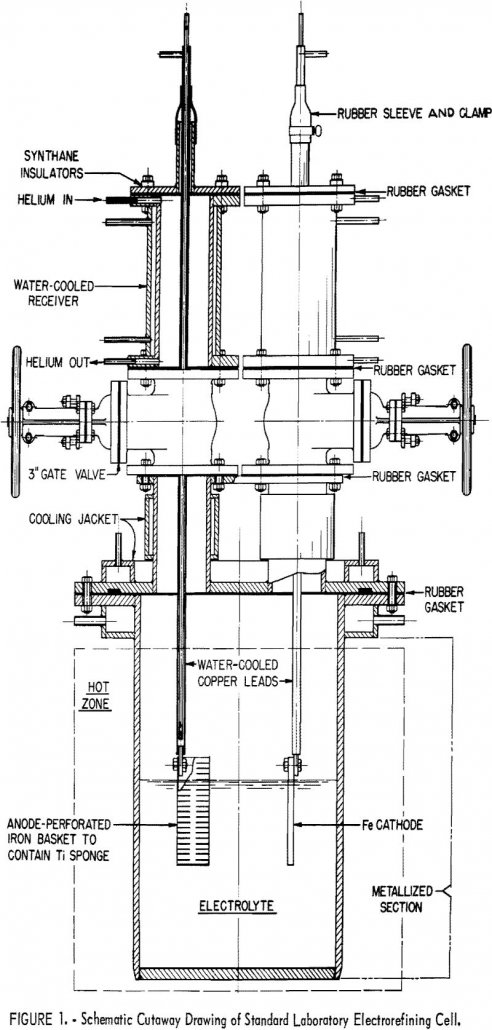
This series of investigations was exploratory in nature, as all variables that might influence alloy-element transfer were not examined. The test show, that, under the conditions described, titanium can be refined almost completely from molybdenum, tin, and zirconium; that refining from aluminum and chromium is only slightly less successful; and that refining from vanadium and […]
Nickel and Cobalt Electrolytic Separation
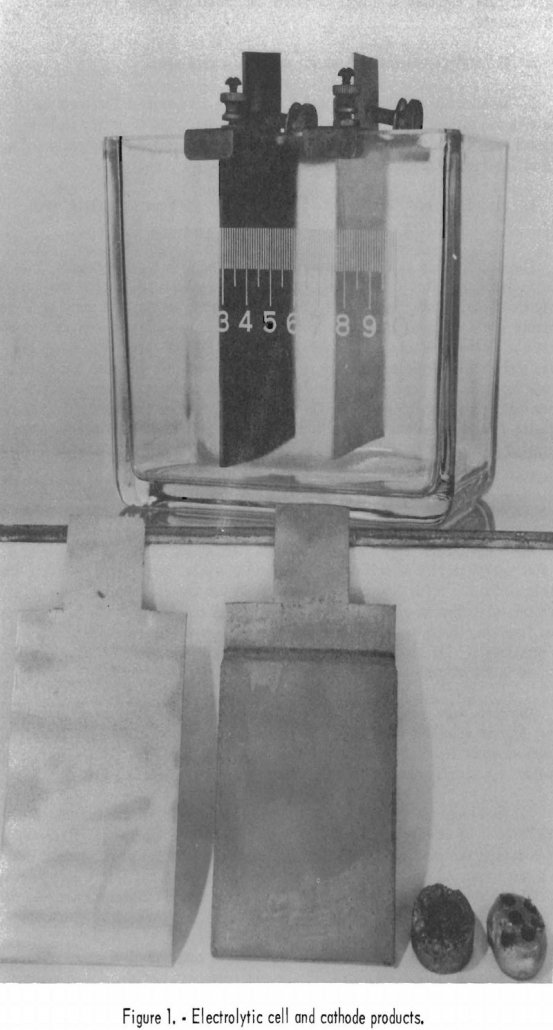
Preliminary work on electrolytes of neutral sulfate, neutral chloride, ammoniacal sulfate, and ammoniacal chloride solutions offered little promise for separating cobalt from nickel by the anodic deposition of cobalt peroxide. Electrolytes composed of synthetic Nicaro liquor, dichromates, cyanides, and phosphates offered little or no promise. An ammonium sulfate electrolyte showed separation of cobalt from nickel […]
Electrorefining Vanadium Scrap

A molten-salt electrorefining process was developed which provides a practical way for reclaiming vanadium from scrap generated in the processing of ductile vanadium into bar, sheet, wire, and other shapes. Refining produced extremely ductile vanadium with a purity range of 99.93 to 99.95 percent. The process was particularly effective in reducing the interstitial elements, carbon, […]
Vacuum Arc Melting
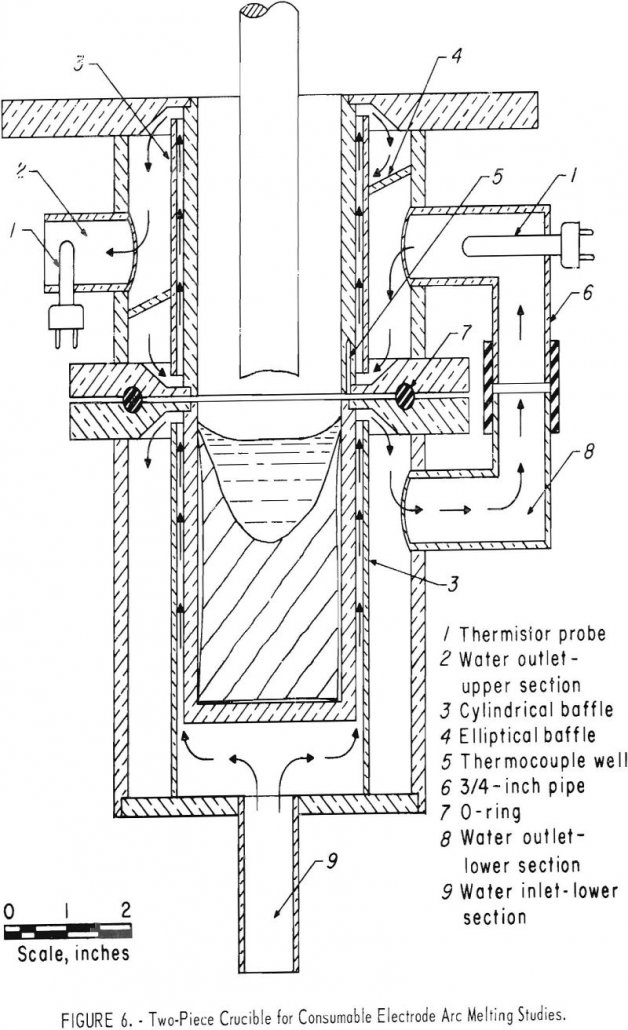
During the past decade, several novel melting and casting techniques including vacuum arc melting, skull casting, Hopkins’ Process or electroslag melting, and electron-beam melting have been developed on an industrial scale. All of these techniques employ a water-cooled crucible. Heat transfer during vacuum-arc melting with water-cooled crucibles has been studied on a theoretical bases, but […]
Electrowinning Rare Earth Elements
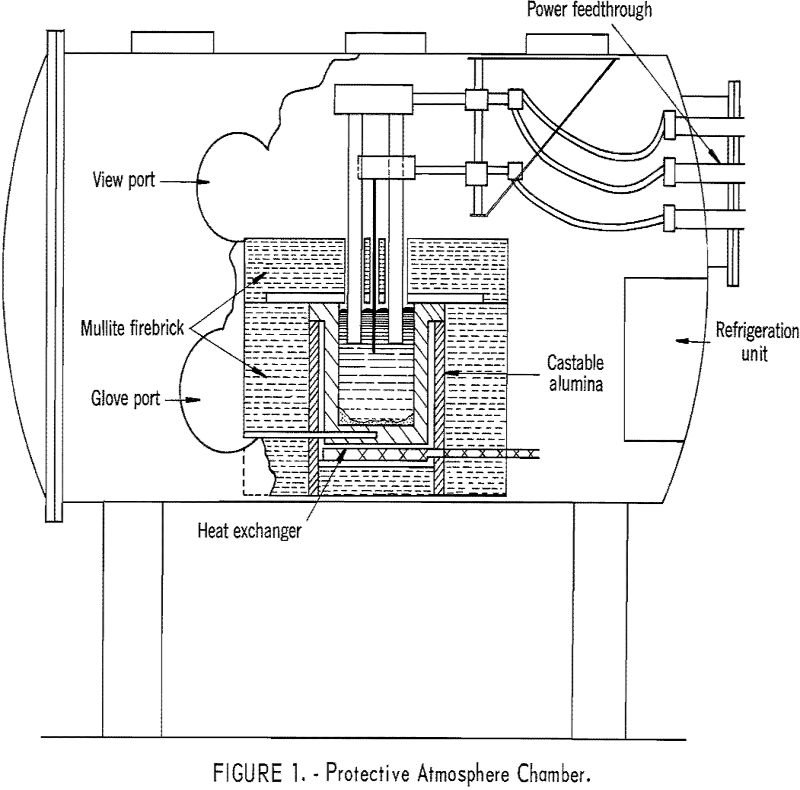
Electrowinning Rare Earth Elements like Neodymium, praseodymium, and didymium, were (obviously) electrowon from their oxides dissolved in fluoride melts. The use of a thermal gradient cell resulted in recovery of nodules of well-coalesced metal with a purity of 99.9 percent. Current efficiencies of up to 83 percent were obtained. Shallow immersion of fluted anodes allowed […]
Electrorefining Vanadium
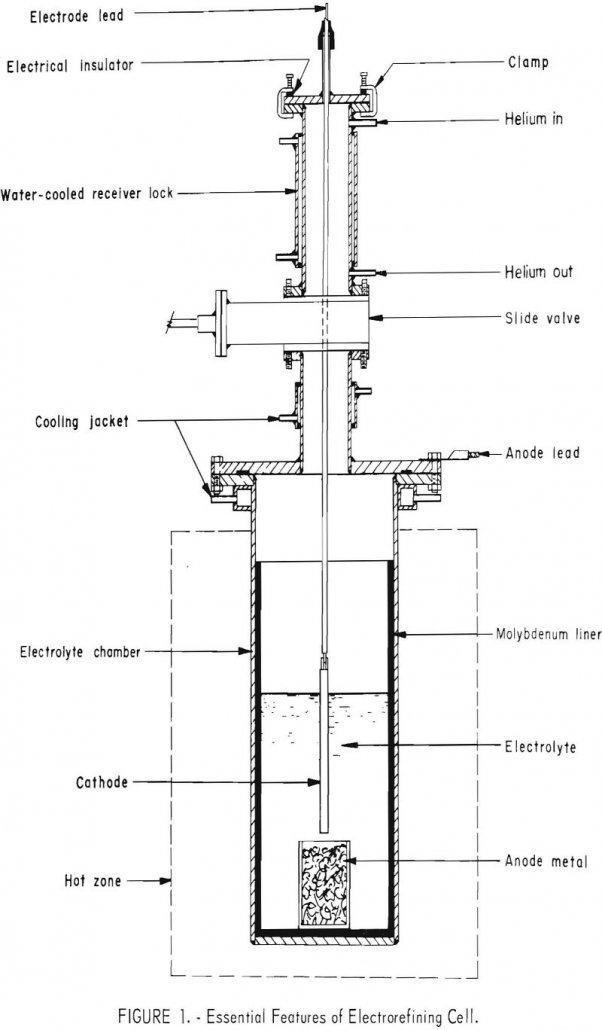
A molten salt, inert-atmosphere electrorefining process was developed for producing ductile vanadium from commercial vanadium. Five different chloride electrolytes were utilized. The chief impurity elements in the commercial vanadium were aluminum, iron, molybdenum, nitrogen, and silicon totaling 8 percent. Refining reduced these elements to produce 99.0 to 99.6 percent vanadium. The process was especially effective […]
Iridium Electrodeposition
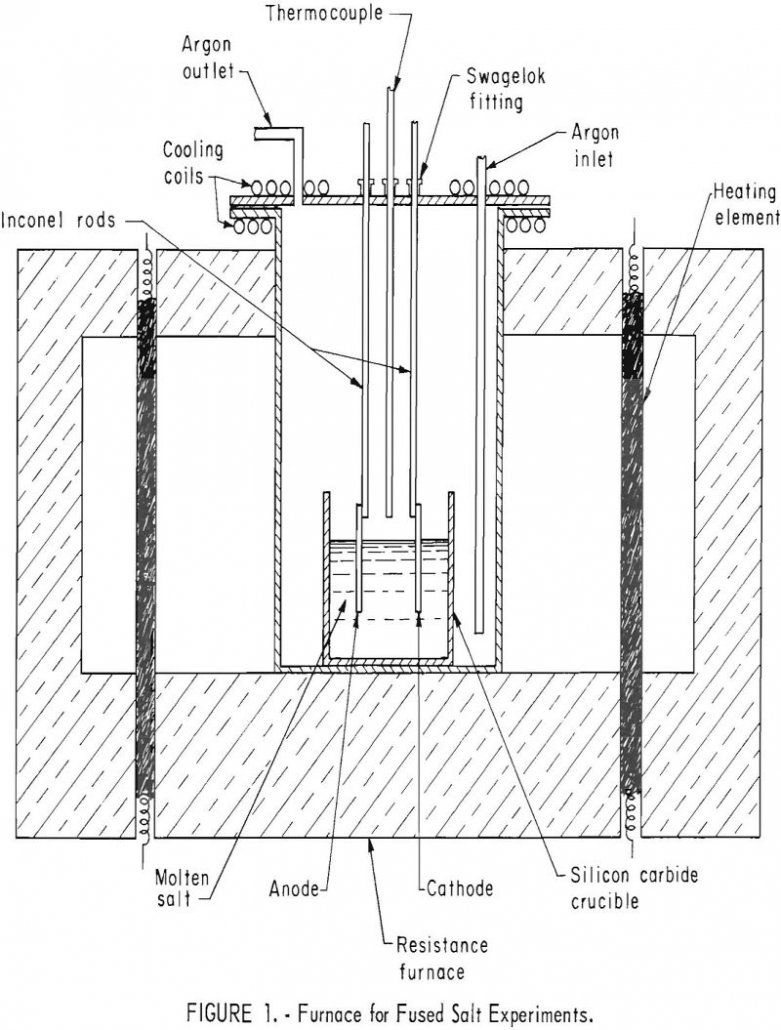
Adherent, coherent electro-deposits of iridium 15 mils thick and alloys of iridium 4 mils thick were formed in argon atmosphere from fused cyanide electrolytes. The use of pure iridium to prevent oxidation appears limited to temperatures under 1,000° C because of volatility of the oxide of iridium. Iridium alloyed with platinum-group metals such as platinum, […]
Electrorefining Yttrium
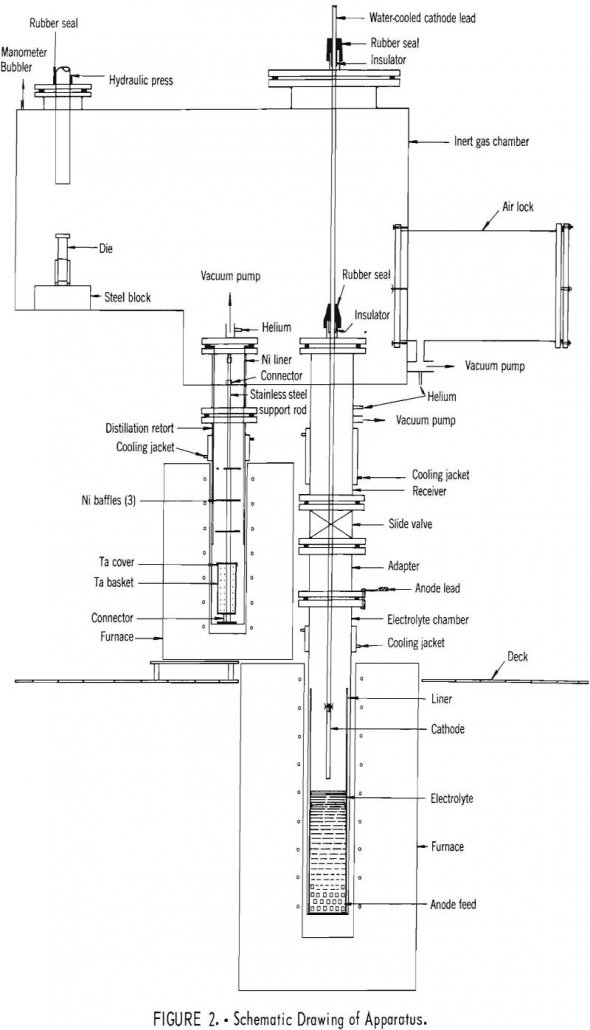
Tests on electrorefining of yttrium were performed in LiCl-YCl3 electrolytes at 710° C, and the deposits were subjected to vacuum-distillation at 880°C to remove salt from the metal. A comparison of the analyses of the anode feed and the product metal indicates that all metallic impurities were substantially lowered, but oxygen was reduced appreciably only […]
Electrowinning Neodymium
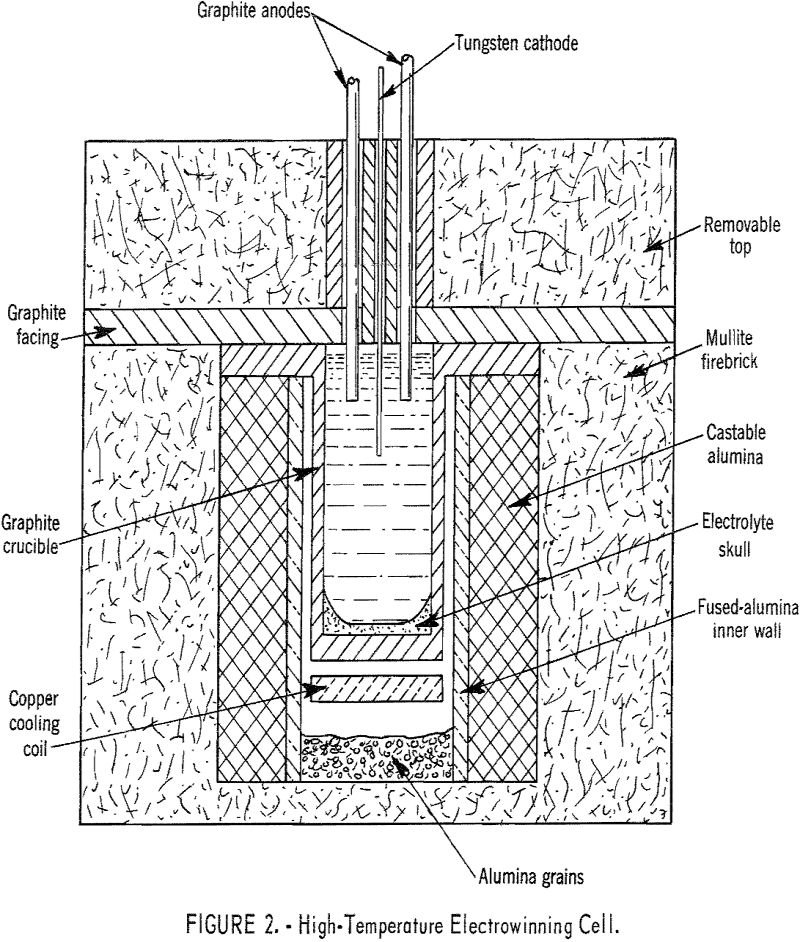
Cells were designed and successfully operated on a laboratory scale for electrowinning high-purity neodymium, praseodymium, and didymium, as well as the higher melting-point metals, gadolinium, dysprosium, and yttrium. The elements were prepared from their oxides, dissolved in fluoride melts, and deposited as liquid metals on a tungsten cathode. Similarly, alloys of these metals and samarium, […]
