Mineral Identification by Spectroscopy

FIG. 35 gives an idea of the spectroscope and of its different parts. P is a flint glass prism, having a refracting angle of 60° and resting on a brass plate fixed on a brass support, S. The brass plate carries the collimator tube C, in the end of which nearest to the prism is fixed […]
PREPARATION OF BASE SOLUTIONS
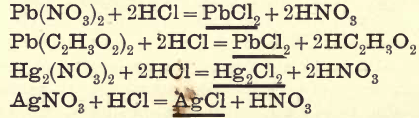
PREPARATION OF SOLUTION FOR BASES 1. Boil the finely-divided substance in distilled water. 2. If insoluble, add ¼ its bulk of strong HCl and boil for two or three minutes. 3. If still insoluble, treat a fresh portion with strong HCl and boil for five minutes ; then add an equal volume of water and warm. The […]
PRELIMINARY EXAMINATION FOR ACIDS
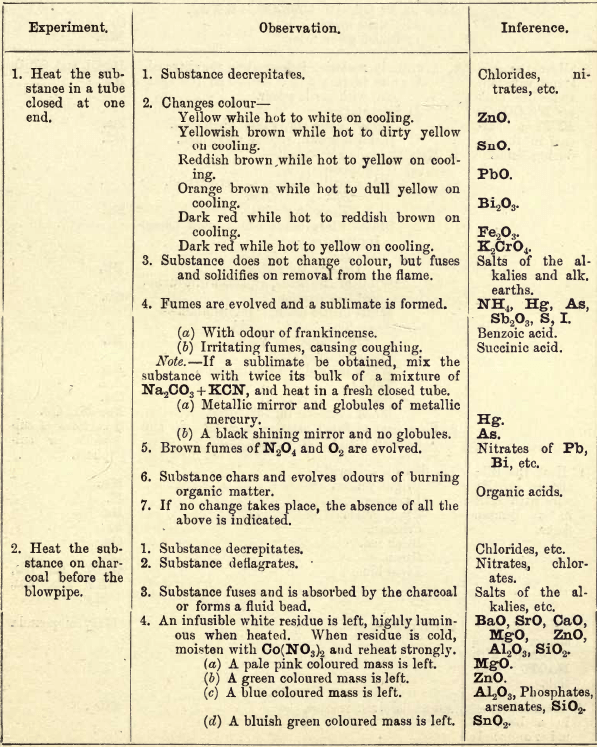
Note 1. Do not boil with Na2CO3 unless necessary. If only alkalies are present it is unnecessary. Note 2. If organic acids and Groups I. and II. or H2CrO4 are present add HCl, and pass H2S before boiling with Na2CO3 to make solution (4). The following should be tested for separately:— HNO3.—Black ring test, with […]
Oxygen Gas
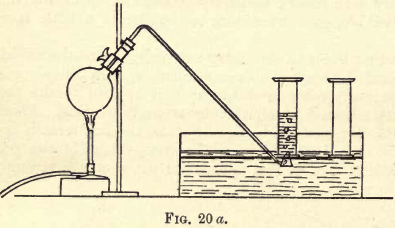
Take a few crystals of potassium chlorate (KClO3), place them in a clean dry test-tube, and heat them gently over a small bunsen flame; the salt begins to split, then fuses. Insert into the test-tube a splint of wood, glowing at the point, but do not allow the wood to quite touch the fused salt. The splint, which […]
Hydrogen Gas
Our Hydrogen Gas experiment involves taking one or two grams of zinc, put them in a test-tube, and add a few drops of dilute sulphuric acid; an effervescence takes place, and bubbles of gas rise through the liquid. Put a lighted taper into the test-tube ; a slight explosion takes place, and you see a momentary flash. […]
Nitrogen Gas
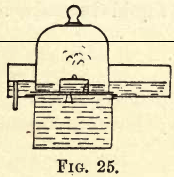
Nitrogen exists in the air, mixed principally with oxygen, so the simplest way to prepare it is to take away the oxygen from the air. Fill the pneumatic trough as before with water, and on the tray stand a bell jar, under which float a small porcelain dish containing a little amorphous phosphorus, as shown in fig. 25. Ignite the […]
Carbon Dioxide Gas
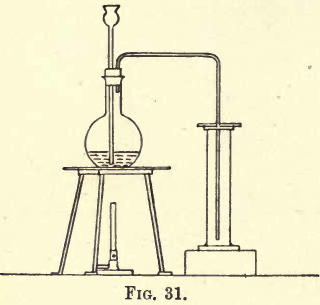
Carbon dioxide is about one and a half times heavier than air, so it can be collected by downward displacement. Fit up a flat-bottomed flask with a thistle funnel and delivery tube, and set up your apparatus as shown in fig. 31. Weigh out about 25 to 30 grams of marble, broken into small pieces, and transfer carefully […]
Nitric Acid Gas
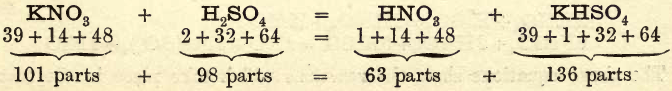
Take a glass-stoppered retort with a capacity of about 250 c.c. and a long beak; clean and dry it. Weigh out 25 to 30 grams of potassium nitrate and transfer it to the retort through either a glass funnel or an improvised paper funnel, taking care that none of the salt adheres to the neck of […]
Nitrogen Monoxide – Nitrous Oxide Gas
This gas can be prepared from ammonium nitrate, which can be made by neutralising dilute nitric acid with ammonium hydrate. Take some of the nitric acid left over from the last experiments, dilute it with a little water, put it in a porcelain basin, and add ammonium hydrate to it, keeping it stirred with a […]
Ammonia Gas
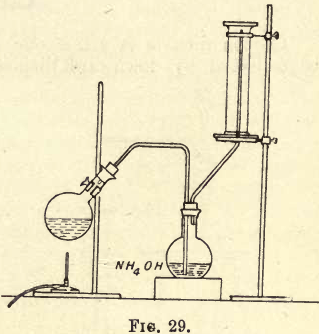
Ammonia Gas is so soluble in water that it cannot be collected as before over water in the pneumatic trough, and as it is lighter than air, fix up your apparatus as shown in fig. 29 and collect it by upward displacement. Mix a little ammonium chloride with an equal amount of quicklime and put it in a […]
