Table of Contents
This is of special interest in hydrometallurgical processing since it provides an upgraded sulfide and rejects iron to solution. Also, the conversion of chalcopyrite is an important process in secondary mineralization during supergene enrichment, and secondary mineralization may occur during long cycles of dump leaching. In deep solution mining, secondary reactions undoubtedly will assume an important role.
Chalcocite is the most abundant mineral of the secondary copper sulfides. It is deposited by various reactions along with covellite, with which it is commonly associated.
ZnS + CuSO4 = CuS + ZnSO4…………………………………………………(1)
PbS + CuSO4 = CuS + PbSO4………………………………………………….(2)
FeS + CuSO4 = CuS + FeSO4…………………………………………………(3)
CuFeS2 + CuSO4 = 2 CuS + FeSO4………………………………………..(4)
5CuS + 3CuSO4 + 4H2O = 4Cu2S + 4H2SO4…………………………………………(5)
Cu5FeS4 + CuSO4 = 2Cu2S + CuS + FeSO4…………………………………………….(6)
At the same time, reaction (5) takes place and is considered undesirable since it increases the required CuSO4 recycle by 20-30%. A fine grind to about 80% minus 15 microns was required to increase the reaction rate, and iron removal was around 50%-70% for chalcopyrite and 80%-90% for bornite.
Experimental
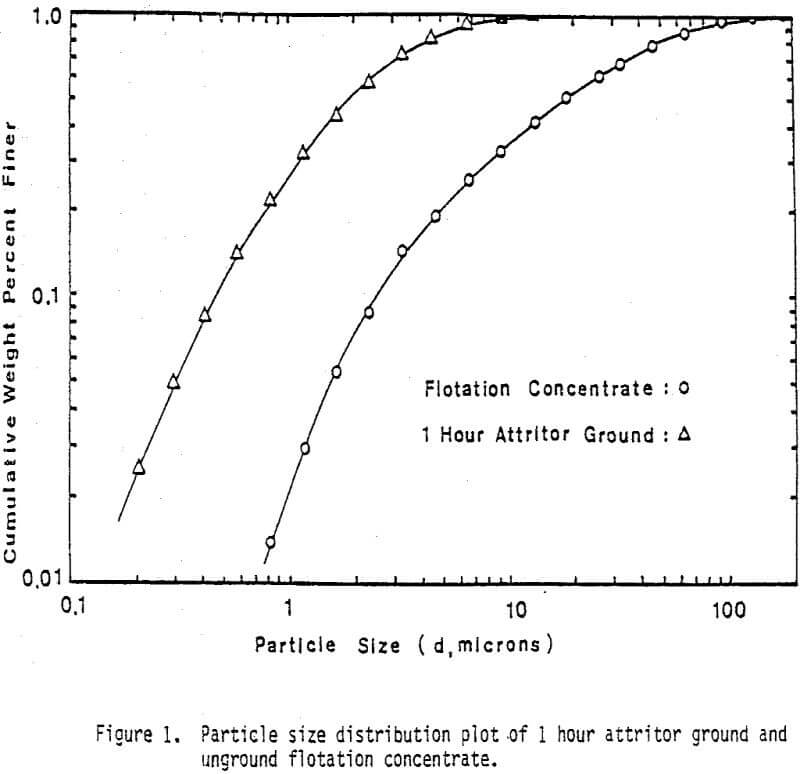
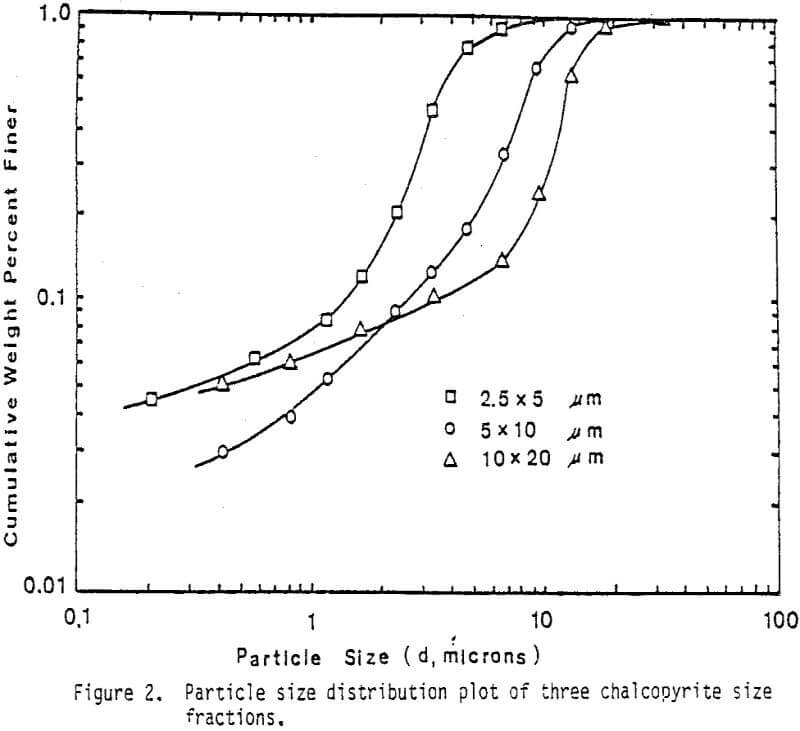
Fine monosize particles, 2.5×5 µm, 5×10 µm, and 10×20 µm, were prepared from Pima concentrate by the Donaldson Company with an Accucut Model B air classifier. Stirred ball milling was carried out in a one-gallon tank Model l-S Union Process Attritor Mill using 15.25 kg of 0.25″ diameter stainless-steel balls. The mill was operated at 340 rpm at a solids-to-pulp ratio of 60%. Particle size-distribution measurements were performed with a Micromeritics Sedigraph 5000D Particle Size Analyzer.
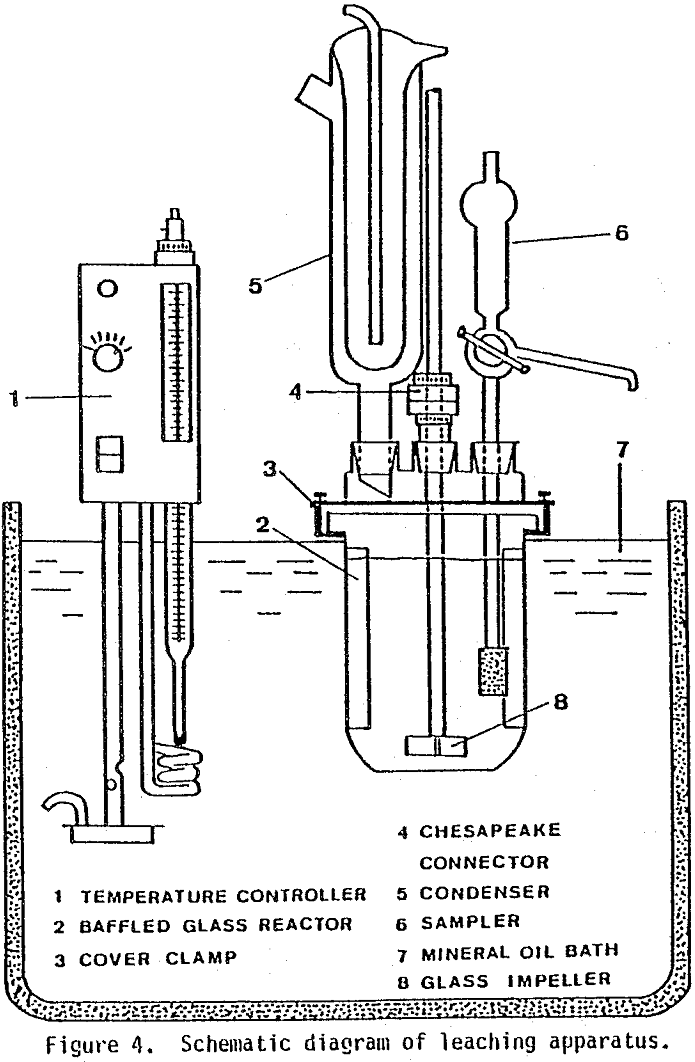
Leaching tests were run in a 1-liter glass reaction. kettle containing three plexiglass baffles cemented with silicone rubber. The removable kettle cover had four ground-glass ports which contained a Friedrichs condenser, sampling device, nitrogen-dispersing fritted glass, and glass impeller. The turbine-type impeller was inserted through the center port by means of a Chesapeake stirrer connection. The entire reaction assembly was clamped into place in an oil bath which was heated with a Haake temperature controller.
Results and Discussion
The chemical conversion of chalcopyrite into copper sulfide with cupric ion under a nitrogen atmosphere may be expressed as,
CuFeS2 + CuSO4 = 2 CuS + FeSO4……………………………………….(7)
6 CuS + 3CuSO4 + 4H2O = 5Cu1.8S + 4H2SO4……………………………………(8)
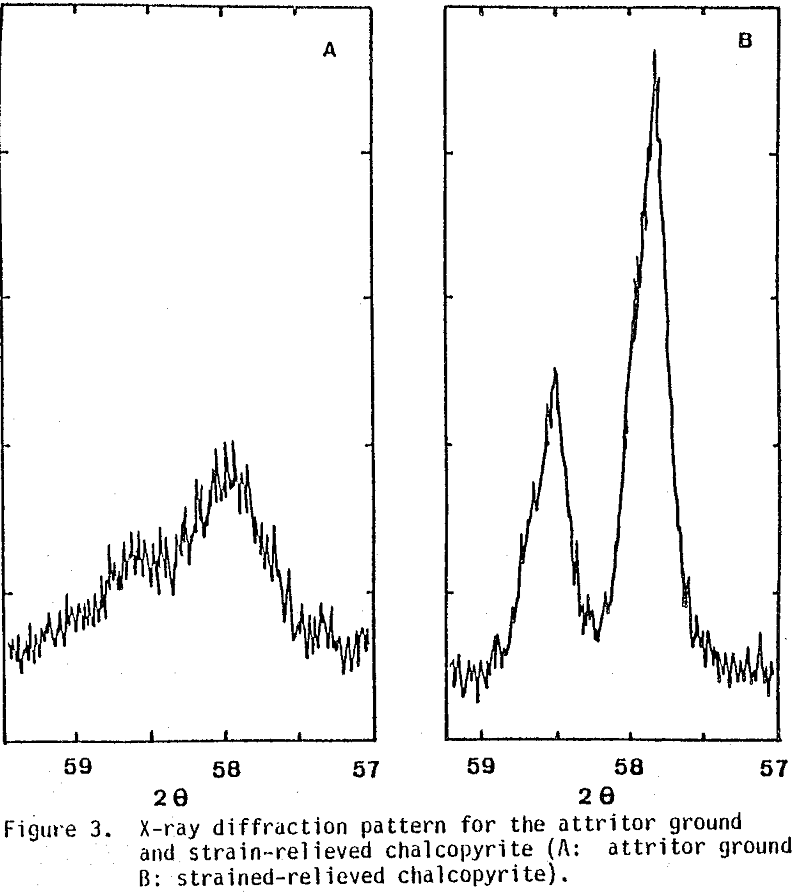
As was discussed before, the above reactions are very slow under normal leaching conditions. Preliminary tests showed that the conversion of 170x 200 mesh (90×75 µm) size was around 4% at 90°C after 100 hours.
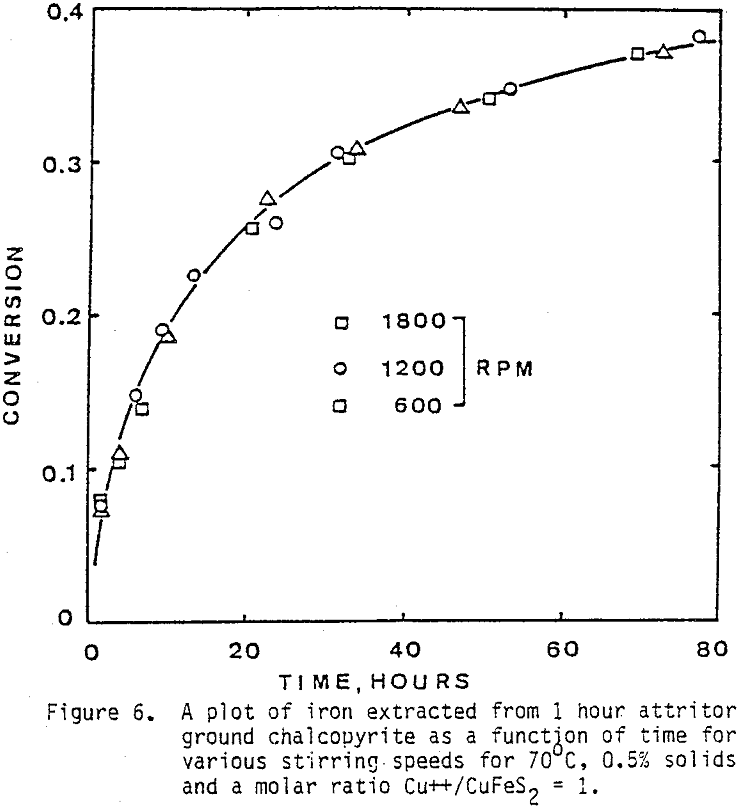
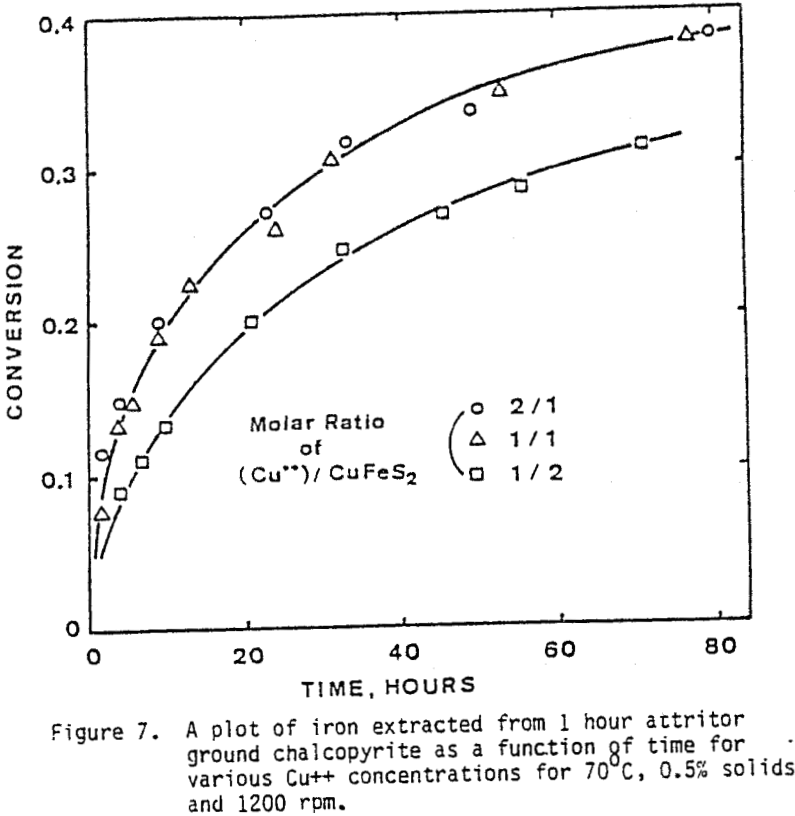
The effect of agitation for three different agitation speeds is shown in Figure 6. Experiments were run with one-hour attritor-ground chalcopyrite, 0.5% solids, molar ratio Cu++/CuFeS = 1.0, ambient nitrogen atmosphere, and 70°C.
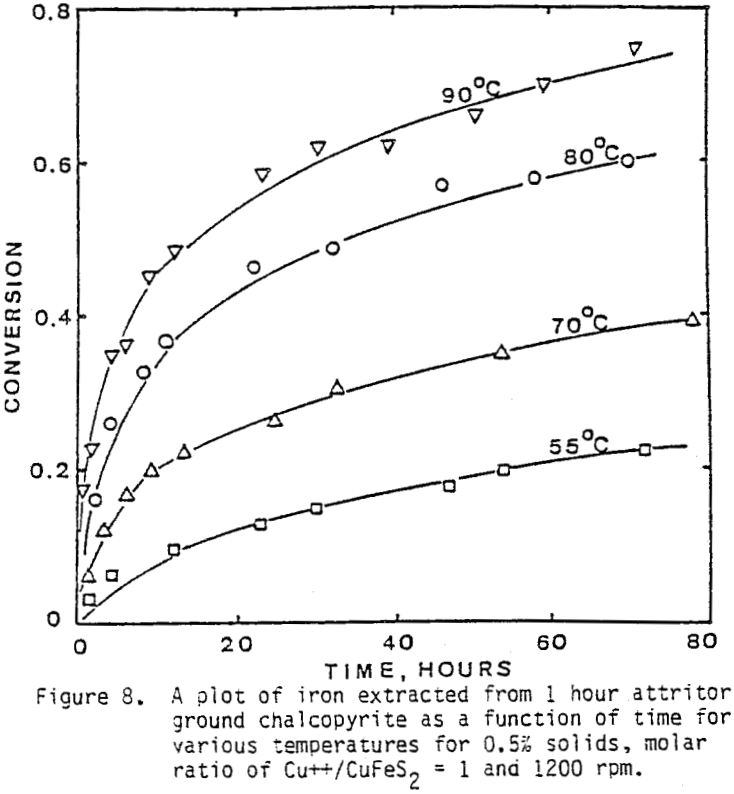
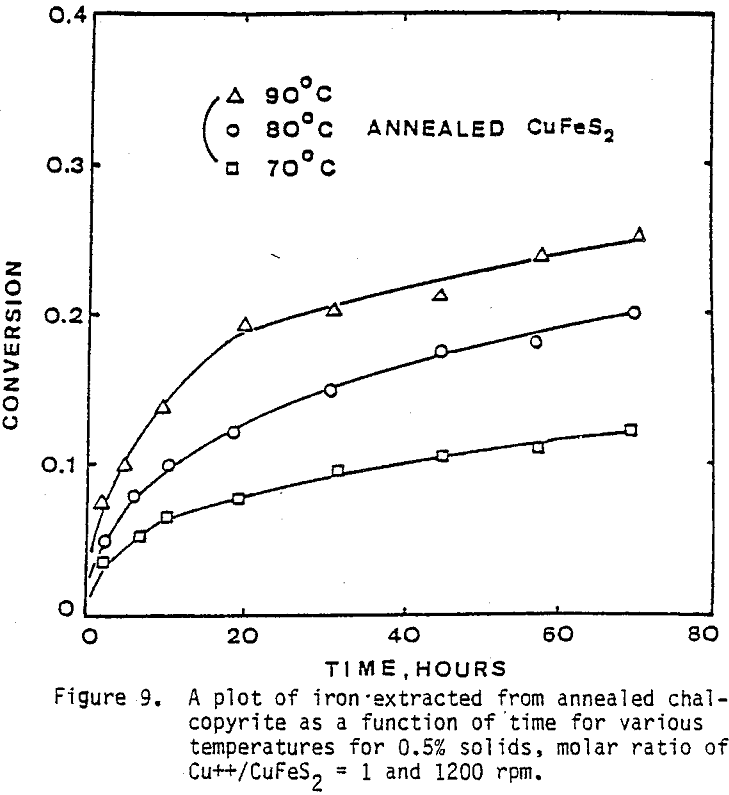
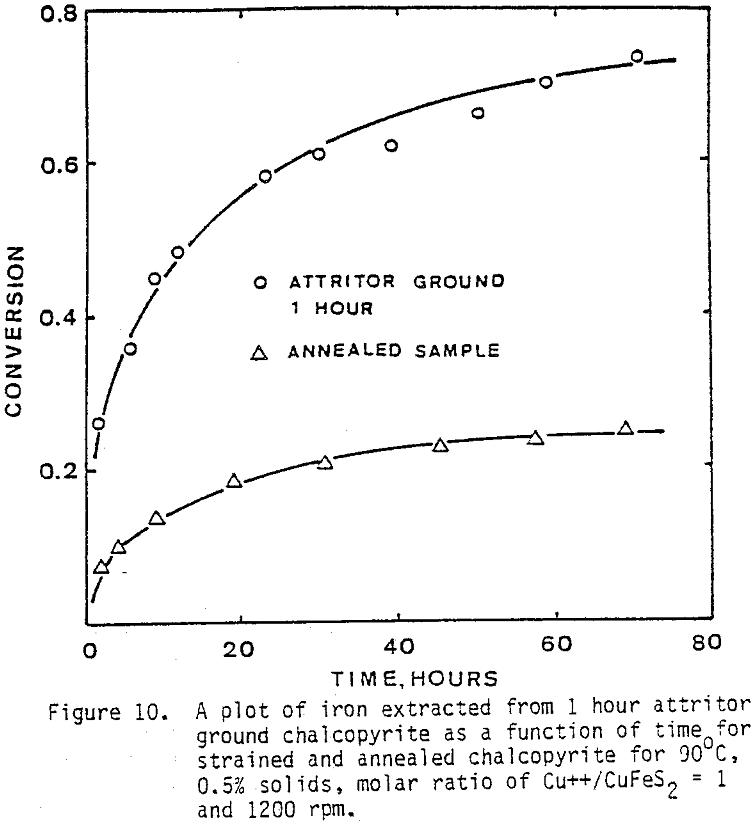
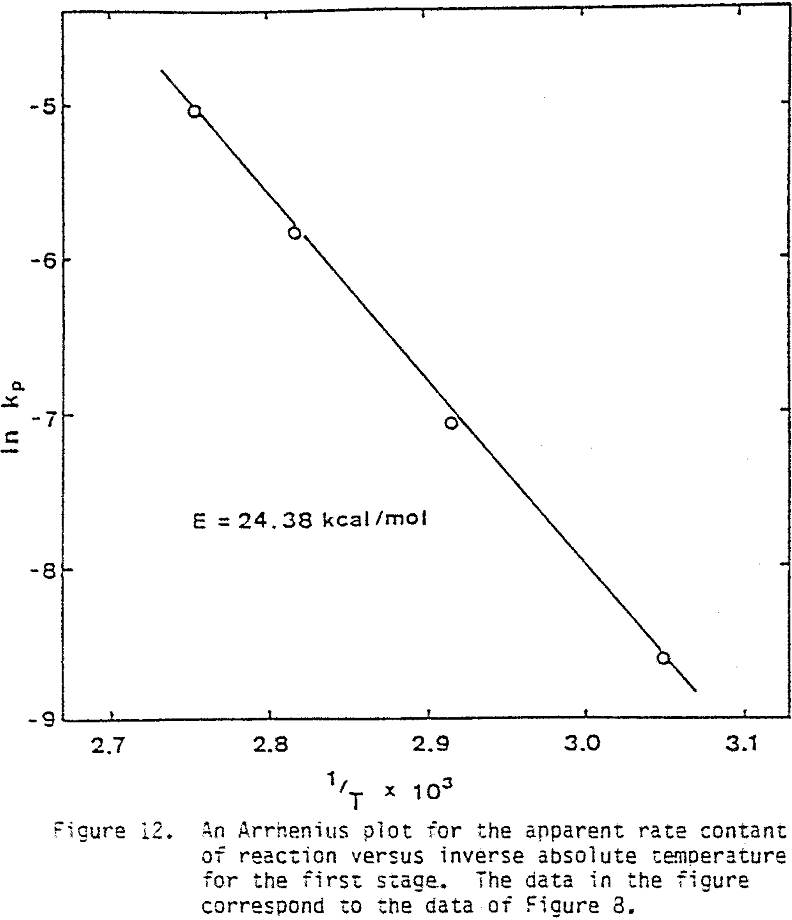
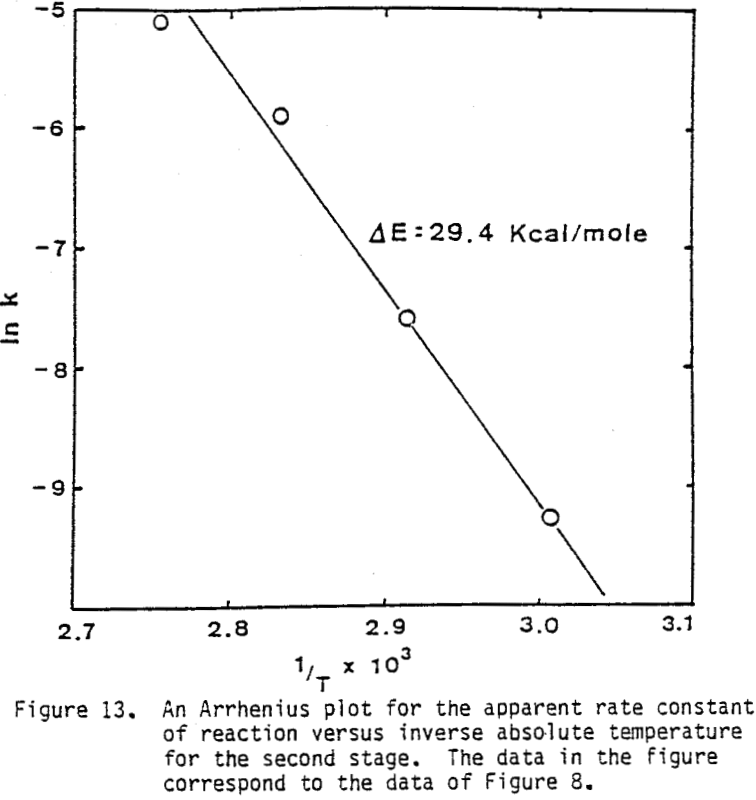
Several tests were performed in order to determine the effect of temperature on the reaction rate. As the temperature was increased the reaction rate also increased, where the effect of temperature on the leaching response of one-hour attritor-ground particles for 0.5% solids, 1200 rpm, and a molar ratio Cu++/ CuFeS2 = 1 is presented.
Kinetics of Reaction
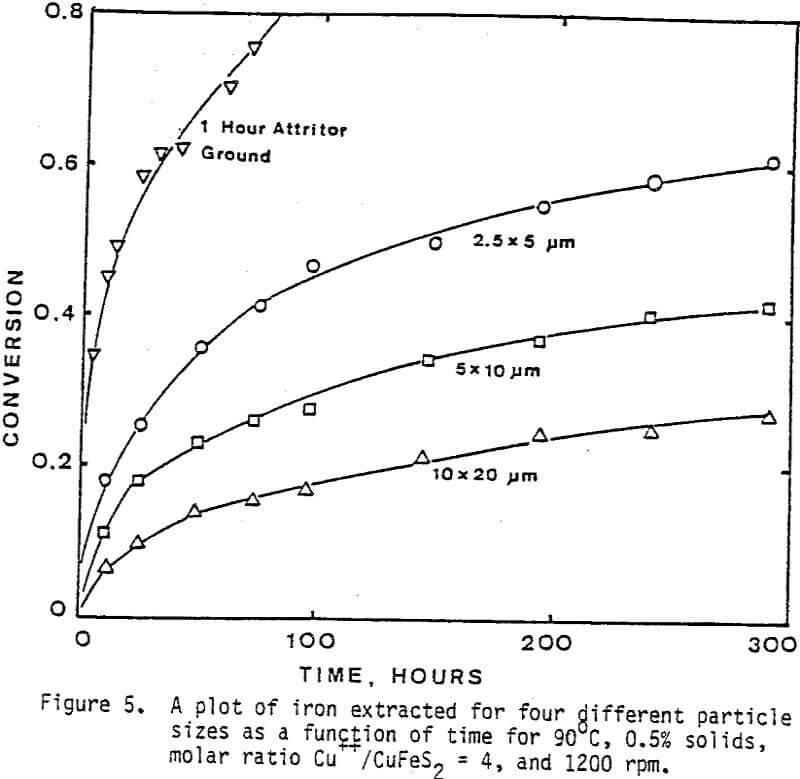
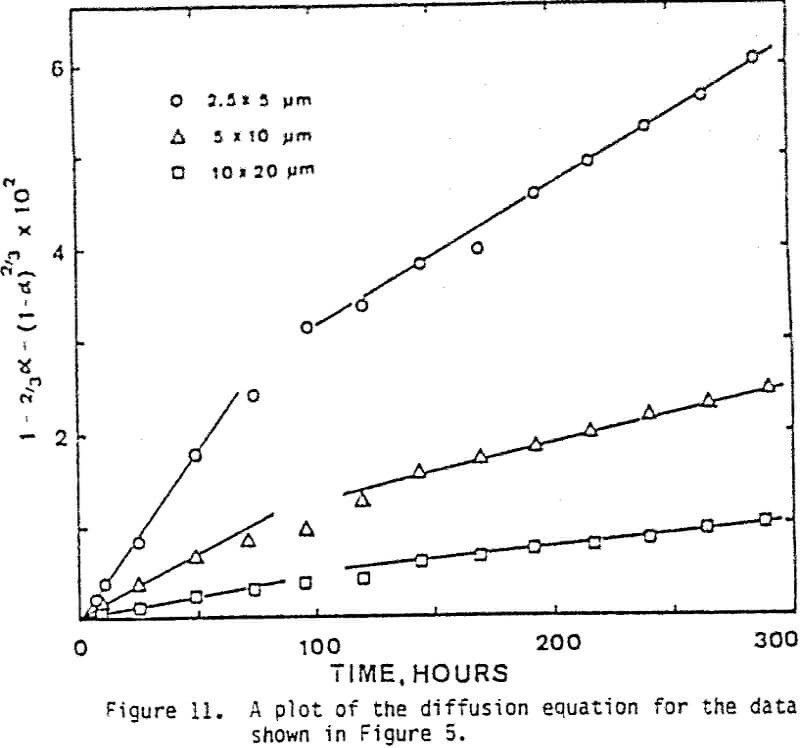
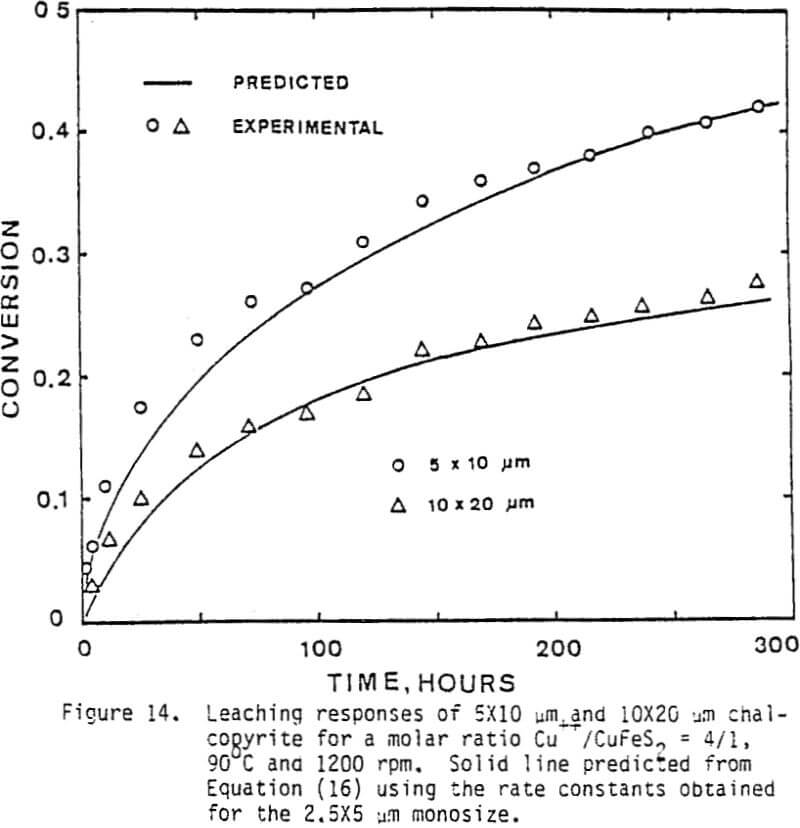
The leaching response of 2.5×5 µm, 5×10 µm, and 10×20 µm monosize chalcopyrite particles are presented in Figure 5, as fraction of iron released versus time plots for 90°C, 0.5% solids, 1200 rpm, and a molar ratio Cu++/CuFeS2 = 4.0.
The total flux may be represented by the equation
dn/dt = 4πr²DγdC/dr…………………………………………………………..(9)
where
n = the numer of moles of chalcopyrite in a particle at time t
r = radius of the particle at any time t
D = the effective diffusion coefficient for transport through the product layer
C = concentration of diffusing species
γ = stoichiometric factor

where
ro, = initial particle radius
ΔC = concentration difference between reaction interface and bulk phase.
Also the number of moles can be expressed as,
n = 4πr³/3v………………………………………………………………….(11)
where
n = moles of unreacted mineral
v = molar volume of reacting solid.