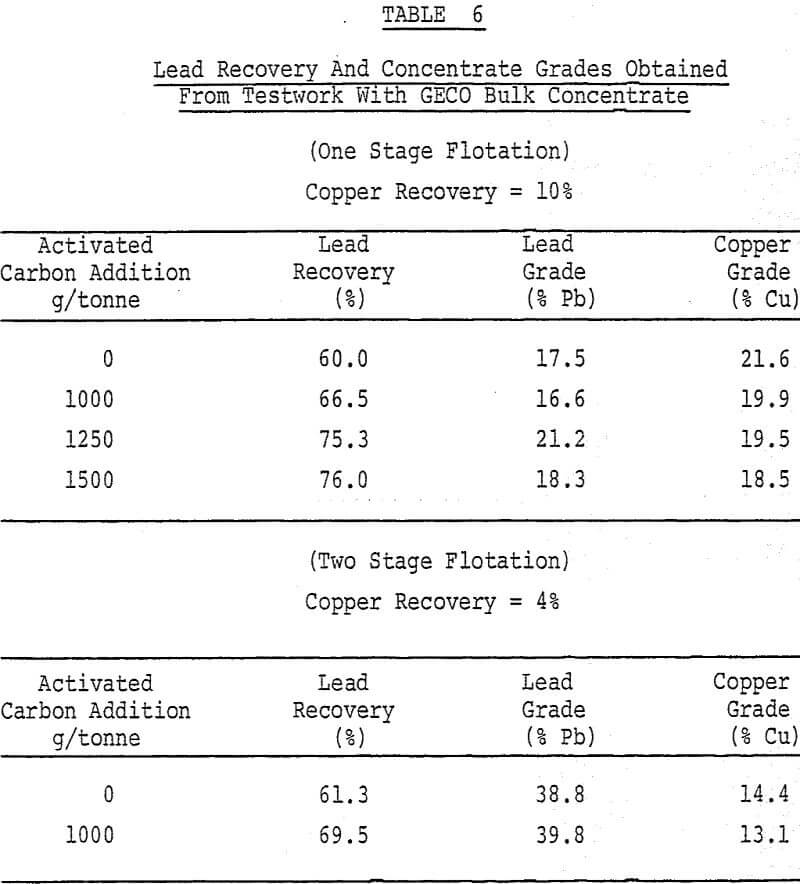
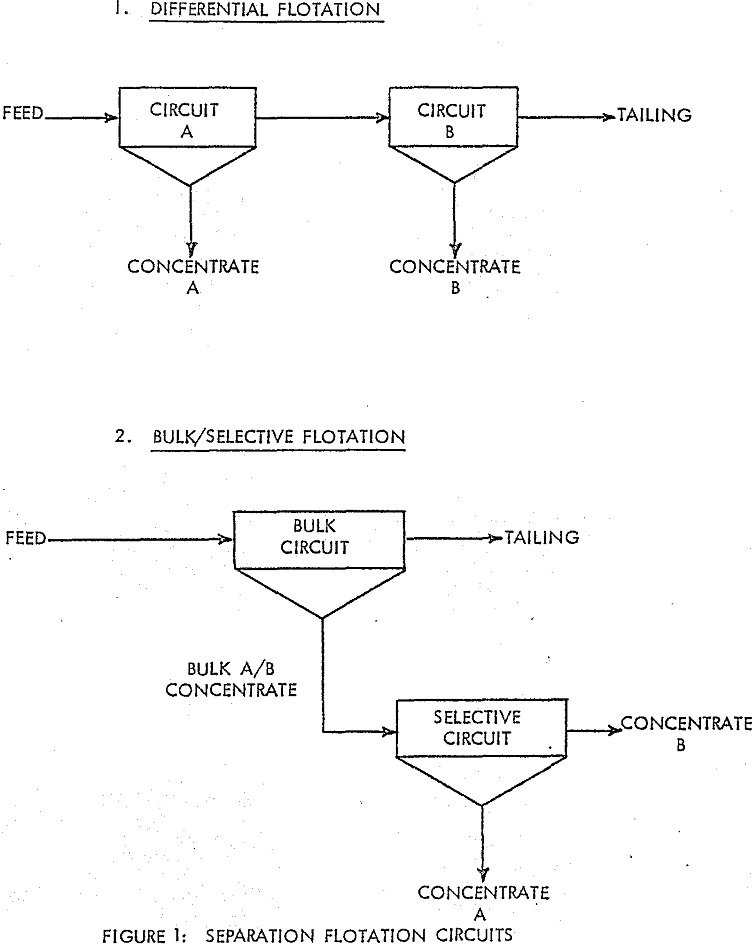
In many multiple-sulphide ore flotation processes, it is desirable to concentrate each of the various valuable minerals into separate products. Although there are a variety of techniques and chemicals which can be employed to produce efficient separation, two basic types of flotation circuits are used: The Differential Flotation Circuit and the Bulk/Selective Flotation Circuit.
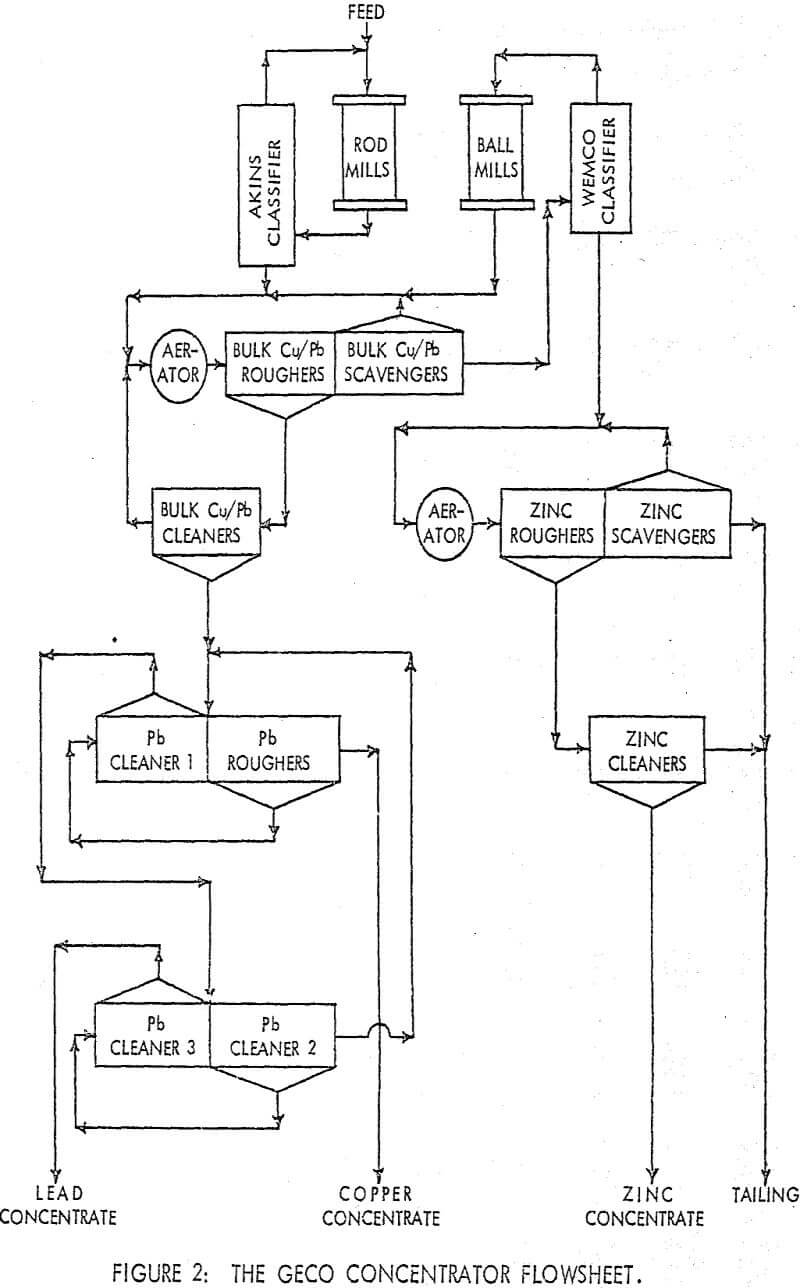
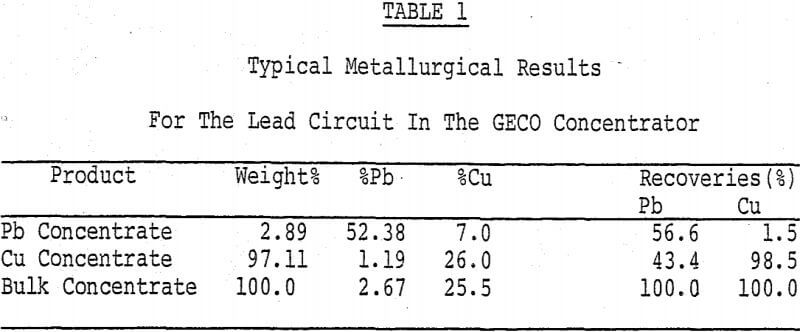
Geco Concentrator Metallurgy
The testwork was aimed at improving selectivity in samples of a copper/lead ore from the GECO mine located at Manitouwadge, Ontario. The basic components of the flotation circuit involve the combination of a differential circuit for copper/zinc separation and a bulk/selective circuit for copper/lead separation. The use of a bulk/selective circuit for removing galena from chalcopyrite is an obvious choice upon examining the relative quantities of the two minerals in the bulk concentrate.
Adsorption of Amyl Xanthate on Activated Carbon
The adsorption tests, using 100 milligram samples of activated carbon were carried out in 50 ml centrifuge tubes completely filled with freshly prepared xanthate solution to minimize the presence of air bubbles. Agitation was provided by rotating the sample in a 12 inch diameter drum at 40 rpm.
The adsorption isotherm demonstrates the high affinity of activated carbon for xanthate, with over 95% removal from solution at initial concentration levels up to 350 mg/litre. At residual concentrations above 150 mg/litre, the adsorption of xanthate is more than one quarter of the weight of the activated carbon.
The inhibiting influence of pine oil, sodium cyanide and pH on xanthate adsorption were also investigated. At pine oil additions of 0.3 to 0.4 mg/litre the adsorption density of xanthate was decreased only 13%. Sodium cyanide caused a greater decrease in density but a limiting value of 70% of maximum was reached at concentrations in excess of 200 mg/litre. Changing the pH from 7.0 to 11.0 caused a drop in xanthate adsorption of about 25%.
Generally activated carbon is considered to be a non-polar adsorbant and yet these results suggest that competition takes place between xanthate and both polar and non-polar adsorbing species. The polar ions, OH- and CN-, both cause a drop in the adsorption density of xanthate, although the limiting effect of cyanide remains unexplained. Activated carbon is known to be an adsorbant for cyanide complexes and since cyanide is present in the GECO system it was important to determine its influence on carbon affinity for xanthate.
Initially xanthate is removed from the mineral surface at a rate which is independent of cyanide concentration. However, at low cyanide levels (50 mg/ litre) after about 1.5 to 2.0 minutes of contact, the adsorption density begins to increase demonstrating the instability of cyanide action.
Action of Activated Carbon on Chalcopyrite and Galena
With activated carbon in the svstem, depression of chalcopyrite is stabilized for a cyanide concentration of 50 mg/litre. Cyanide has little depressing action on galena as would be expected. However the results shown in Figure 9 indicate that activated carbon at high addition rates tends to depress galena by a small amount. Only at rates in excess of 2000 g/t is recovery substantially retarded, being 76% at 3000 g/t and 64% at 4000 g/t.
Thus, the fact that galena is depressed by activated carbon alone and the fact that the depression of chalcopyrite by cyanide is intensified in the presence of activated carbon, suggest that xanthate may actually be desorbed from the mineral surface by the carbon itself.
The role of activated carbon in improving frothing conditions cannot be overstated. Severe problems have probably been experienced in all concentrators at some time with controlling the froth on flotation machines. When added in excess, due to failure or error, most frothers will cause a continual build-up in froth until a “runaway” occurs. Sliming conditions also cause upsets since the tiny hydrophobic particles by themselves can promote froth formation.
Geco Ore Testwork
An increase in the quantity of sodium cyanide produces a steady increase in the recovery of lead at a constant recovery of copper up to an addition rate of 100 g/tonne of ore. The selectivity improves only marginally at higher cyanide levels.
It is also evident that activated carbon improves the selectivity between lead and copper up to 150 g/tonne of ore. The variability of the bulk concentrate produced in this program was considered too great however to define the exact effect of carbon additions.
Two points are important to consider in this analysis. Firstly, it is evident that lead recovery drops as the addition of activated carbon increases (at 750 g/tonne, lead recovery equals 78.5% as opposed to 83.0% with no carbon addition for two minutes flotation time). Thus, it will be necessary to modify the operation of the flotation machines to increase the rate of removal of lead concentrate. Secondly if the copper recovery to copper concentrate increases by only 0.5%, the profit improvement noted above will be increased by 50%.