Table of Contents
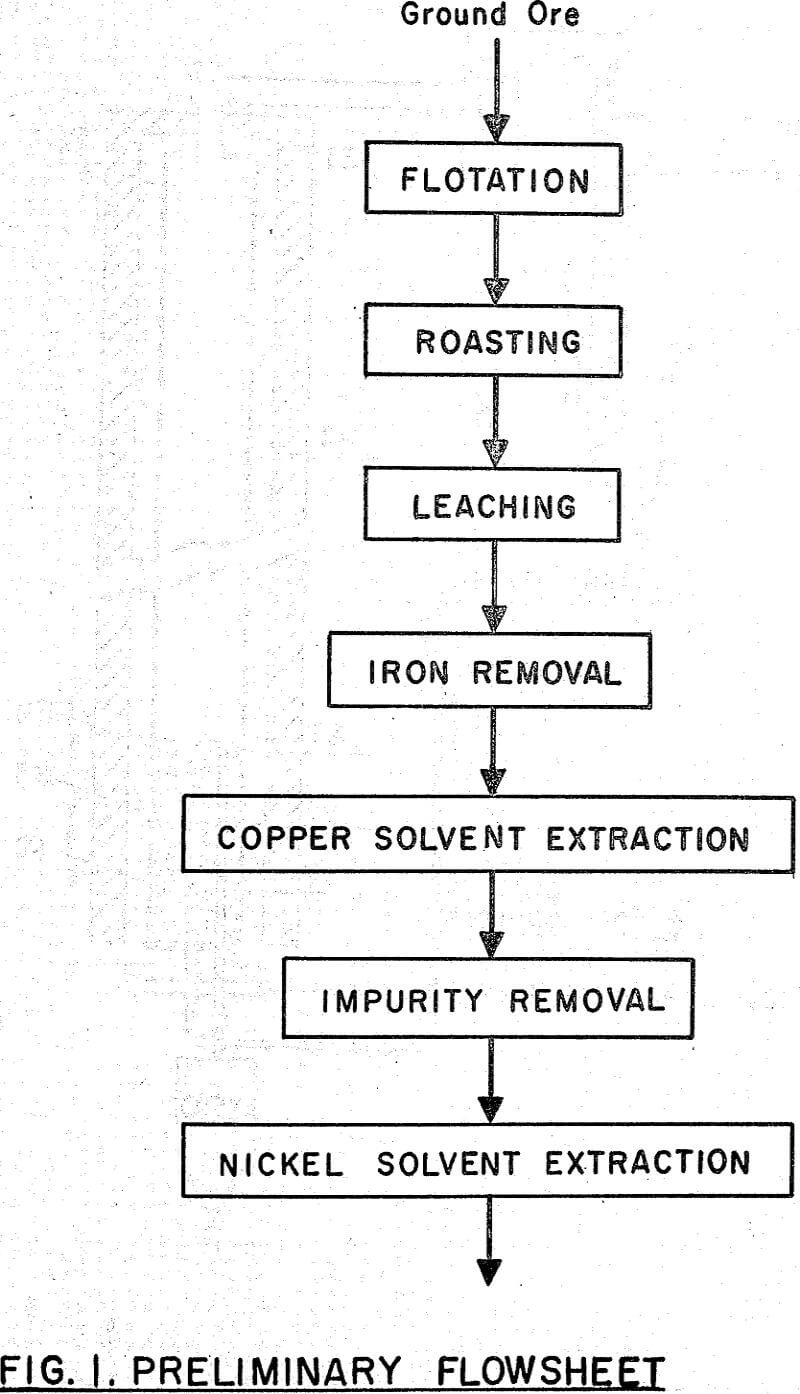
A nickel-copper sulphide concentrate was treated in a pilot plant at Warren Spring Laboratory during 1961, by a hydrometallurgical roast-leach-solvent extraction process devised to cleanly separate the metal values, and produce acid sulphate liquors containing the metals.
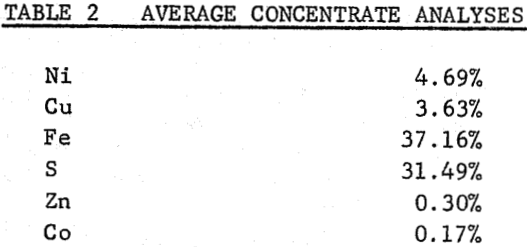
It is shown that 86 percent of the nickel and 95 percent of the copper could be leached from the calcine, after a sulphatizing roast on finely ground flotation concentrate. Iron was removed from the leach liquor by limestone neutralization with a small loss of values. Copper and nickel were separated by solvent extraction with naphthenic acid in kerosene after partial neutralization of the iron-free liquors with ammonium hydroxide. Hydrogen sulphide and chlorine were used to remove zinc (and residual copper) and cobalt impurities respectively.
Flotation
The ore was an altered basic rock containing concentrations of magmatic sulphides. The sulphide phase contained the copper and nickel values and consisted of the minerals pyrite, pyrrhotite, pentlandite and chalcopyrite. The pentlandite often occurs as fine exsolution intergrowths within the pyrrhotite with a liberation size of the order of 10-20 microns.
A 100 lb/hr flotation pilot plant at Warren Spring provided a bulk sulphide feed for the process under investigation.
The -10 mesh feed was ground in a 12-inch diameter by 24-inch rod mill, conditioned for 5 minutes with 1.5 lb/ton of soda ash and 0.4 lb/ton of amyl xanthate, and floated for 20 minutes in 8 Denver No. 5 cells. Flotation contact time was approximately 20 minutes.
Roasting
Initial studies on Na2SO4 as an additive to sulphate nickel in nickeliferous pyrrhotite were made by Sproule. Thornhill developed at Falconbridge Nickel Mines a one-step sulphation technique ensuring intimate contact of the Na2SO4 and the sulphide minerals. Thornhill’s sulphation roasting technique appeared to be applicable to the bulk sulphide flotation concentrate obtained in this investigation,although this concentrate differed from that used by Thornhill and Sproule in two respects – firstly, it had a higher nickel and copper content and secondly, the nickel was present as finely disseminated pentlandite and not as nickeliferous pyrrhotite.
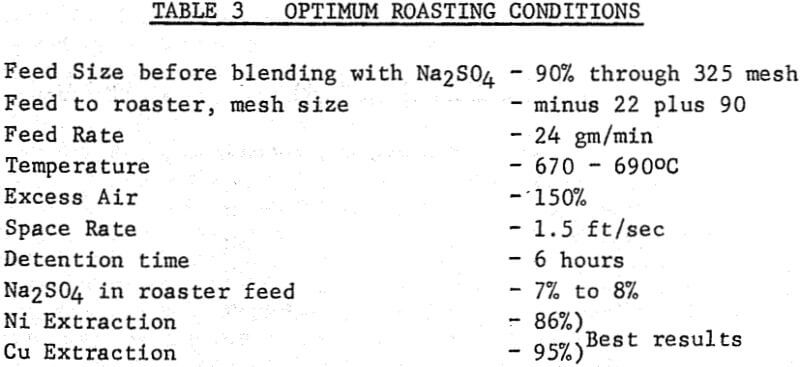
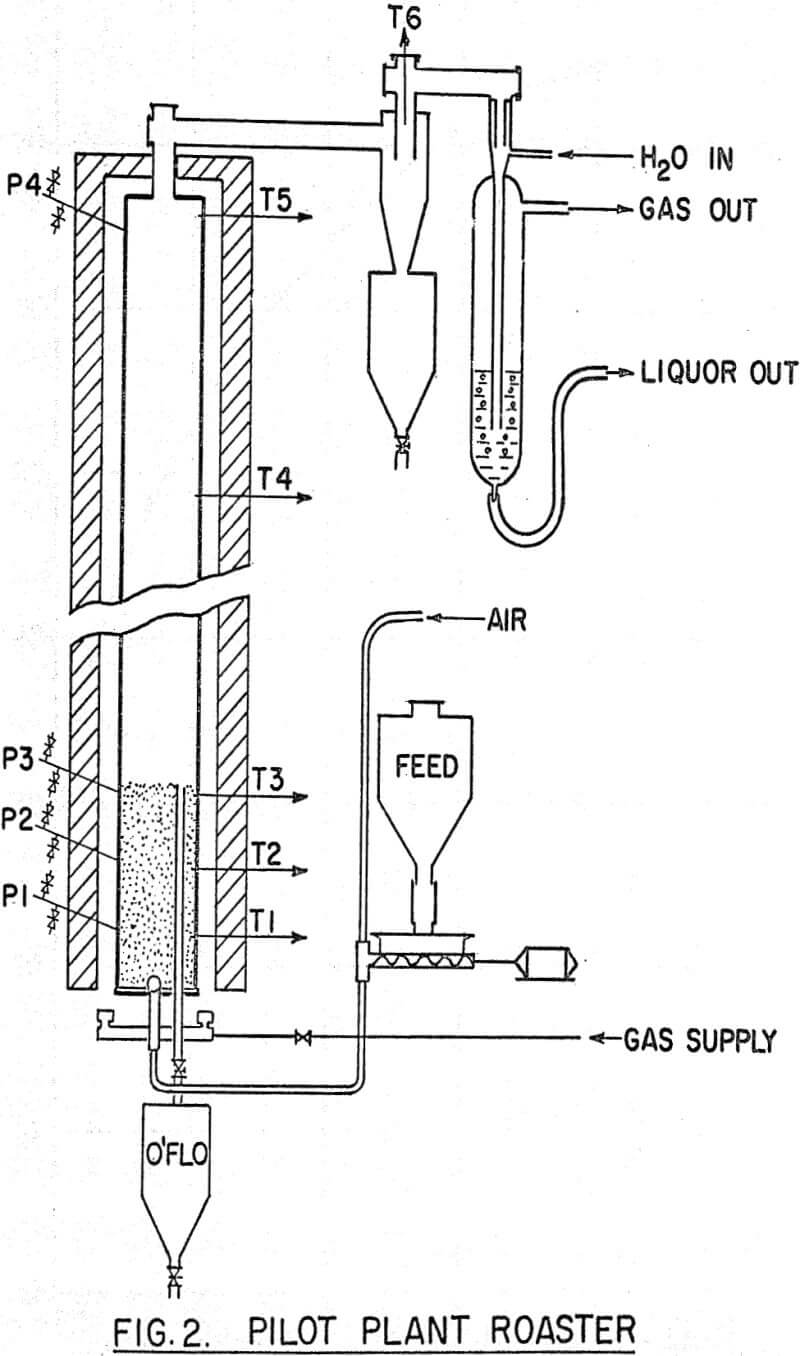
Dried sulphide concentrate was fed to a 4-inch diameter by 9 foot long fluidized bed roaster via a screw feeder, the end of which was swept by the main fluidizing air which entered the bottom of the bed. The exit gases passed through a cyclone and wet scrubber before being discharged to atmosphere. The bed depth (expanded) was normally kept at three feet but could be varied by altering the height of an overflow discharge pipe. A series of manometers registered the differential pressure at various points in the bed and freeboard, and a multi-point instrument recorded bed and roaster gas temperatures. Effective control of bed temperature was obtained by varying the fuel gas supply to four burners located in the annulus between the external walls of the reactor and the brick setting. The normal dry feed rate was 24 gm/min which gave a detention time of approximately six hours.
It was not possible to maintain a well fluidized bed with fine, damp, feed containing Na2SO4 because agglomerates formed in the bed. Close attention to feed preparation overcame this difficulty. The procedure finally adopted was to mix the sulphide filter cake from flotation with a concentrated solution of Na2SO4 in a steam-jacketed pug mill. The slurry moisture content was gradually reduced by evaporation from about 35% to 10%.
The amount of Na2SO4 in the feed was the most important variable in nickel sulphation with nickel extraction increasing as the amount of Na2SO4 in the feed increased. It was not possible to operate the roaster for long periods with feed material containing more than 8% Na2SO4 as caking of the bed could not be prevented. The percentage weight of calcine obtained as a bed product increased with the Na2SO4 content of the feed, so that with material containing 6 to 8% Na2SO4, over 90% of the Ni and Cu was recovered as a bed product, about: 7% in the cyclone and only about 0.5% in the wet scrubber.
Leaching and Solution Recovery
The recovery of values from the roaster calcine by leaching depended largely on the degree of sulphation obtained during roasting. Some slight improvement in copper recovery was obtained by acid leaching, but there was no difference in the amount of nickel recovered. Leaching rates were increased by raising the temperature to 80°C. At this temperature, a one-hour leach time was optimum.
The leaching and solution recovery circuit consisted of a table feeder discharging into a PVC lined Denver conditioner, followed by a water washed 1 ft² rotary drum filter unit, a second lined conditioner and a 4 ft² drum filter unit. The conditioners were arranged to give 1.5 hours contact time in each stage. Leach pulp was heated to 80°C in each stage by live steam injection. Water was used for leaching.
Iron Removal
A synthetic liquor containing 6.1 gm/l iron, 21.4 gm/l copper, and 16.2 gm/l nickel, all as the sulphates, was used for initial tests. With pure calcium carbonate and a temperature of 70°C, a considerable portion of the copper, but little of the nickel, (less than 1%) was occluded in the iron cake if more than 90% of the iron was precipitated. An excess of about 3 gm/l calcium carbonate over theoretical was required. Aeration times and CaCO3 requirements were reduced by operating at the higher temperature of 80°C. At least 60 minutes aeration was required for gross iron removal, but the iron in solution is still decreasing even after two hours.
The iron cake was acid washed to recover copper. It was found that a 1% H2SO4 wash reduced copper losses to 2% of the entering copper in the feed. 90% of the iron could be discarded in the washed cake, while the remaining 10% in the acid washings could be recycled with the recovered copper.
Copper Solvent Extraction
The separation of copper from nickel is based on the fact that there is an appreciable difference in the pH at which the two metals are extracted. Copper can be extracted, with very small amounts of nickel, at pH 6.0 to 6.5, while nickel is extracted from the copper raffinate at pH 8.0 to 8.2. These pH values refer to the pH of the aqueous phase entering solvent extraction; the corresponding equilibrium pH values are approximately 4.7 (copper) and 6.2 (nickel).
Preliminary laboratory experiments were carried out to obtain data on the design of the solvent extraction circuit for the pilot plant. The effect of pH on the separation and recovery of copper and nickel using a 1.0 molar kerosene solution of naphthenic acid (Shell N.A. SP230) is given in Table 6A.
Energy requirements and contact times for extraction and stripping were determined using a baffled 9 inch diameter by 12 inch high PVC vessel. The design of the turbine impeller used for stirring and the technique for scale-up were similar to that described by Ryon et al. It was found that with an impeller speed of 400 to 450 rpm and a ratio of organic:aqueous of 2:1, the extraction of copper and nickel from aqueous solutions containing 10 gm/l of the metal was complete in 3 minutes and 1 minute respectively.
The overall metallurgical balance indicated that 96% of the Cu and 1.3% of the Ni was extracted with an ammonia consumption of 0.606 lb NH3/lb of Cu. During several 8-hour periods, however, 98% of the Cu was extracted with only 1% of the Ni. Approximately 80% of the Cu was extracted in stage 1 and 18% in stage 2 while similar amounts of Ni (0.5%) were extracted in both stages. 98% to 99% of the Cu and approximately 60% of the Ni in the loaded solvent was recovered in the single stage stripping circuit. The acid value of the solvent was 1.09N before the extraction run, and 0.978N at the end of the run.
During extraction the organic loading did not exceed 10 gm/l. A maximum operating loading of approximately 16 gm/l should be possible with a 1.0 molar solvent, although extraction contact times might then have to be increased.
Nickel Extraction
In order to investigate the possibility of reducing the solvent loss, equipment for retreating the raffinate from the second extraction stage was included. This involved acidification of the raffinate in a third mixer-settler unit and recycling the organic (pure kerosene at the start). Before discharge, the aqueous phase from this treatment was passed through a pebble bed and another settler. It was thought that solvent droplets which failed to separate in the first settler would coalesce into larger particles on passing through the pebble bed and separate in the final settler.
The alkali used in nickel extraction was the same as that used for copper extraction (7N NH4OH). Initially, the loaded solvent was stripped with dilute H2SO4, giving a NiSO4 product. When sufficient of the NiSO4 product had been collected, H2SO4, H3BO3 solution and Na2SO4 solution were added to make a stripping solution simulating return tankhouse liquor.
The plant was operated continuously for approximately 78 hours at an extraction feed rate of 36 litres/hr. During operation the alkali flow rate was controlled to give pH’s of 8.2 and 8.0 in the feed to the first and second extraction stages respectively. The corresponding raffinate (equilibrium) pH’s were 6.8 and 6.5 respectively. The solvent flow rate was controlled to give a ratio of aqueous : organic such that the maximum solvent loading was 13 to 16 gm/l Ni. Approximately 80% of the nickel entering each extraction stage was recovered so that the overall nickel extraction was 96%. A third stage would probably have increased extraction to 99%.
Possible Plant Flowsheet
Comminution and flotation are conventional.Roasting would be done in a spray-fed fluo-solids roaster with the Na2SO4 added in the solution. The largest loss of nickel values occurs here because of incomplete nickel sulphation. Regrinding the concentrate to all -325 mesh might improve recovery. Further studies have shown that a secondary roast, with air at 650°C, improves the nickel recovery and decreases iron solubility.
In leaching, the main problem is adequately handling a mixture of coarse solids and fine slime at high temperature under corrosive conditions – primarily an equipment problem.
The solvent extraction of copper, followed immediately by nickel solvent extraction will minimize solvent loss. The circuit would be a simple mixer-settler installation with provision for acid treating the organic. Recent studies have shown that the direct addition of alkali to the aqueous-organic mixes reduces the formation of insoluble precipitates. Current Russian papers indicate solvent extraction is now in use in the Servo-Nickel Combine in Russia, utilizing the method mentioned above under iron removal (e.g. exchange of a more acidic metal from the aqueous for a less acidic one in the extracting organic).
The sulphide removal step for zinc and copper has been moved into the nickel electrowinning circuit so that any dissolved lead from the linings of the electrolytic cells will also be removed simultaneously. In this case, nickel carbonate would have to be used as the control alkali. A simple precipitation with filter pressing and washing will remove the impurities. Some provision for H2S recovery (vacuum extraction, heating) will be required to minimize costs.