Commercial copper SX reagents do not exhibit perfect selectivity. From typical acidic feed solutions, the most significant co-extracted impurity is ferric iron. The copper/iron extraction ratio for a given reagent operating in a countercurrent circuit will vary significantly with reagent content of the solvent, composition of the feed solution, organic/aqueous flow ratio, temperature, mixer retention times, mixing intensity, etcetera. For LIX 64N this ratio ranges from greater than 200/l (good) to less than 100/l (bad). These numbers are not always directly comparable with those obtained by batch laboratory contacts of solvent and feed.
Iron extraction, like that of copper, is reversible, and the iron reports to EW in the strip liquor. In EW, the ferric iron is reduced to ferrous iron at cathode surfaces by 1) direct cathodic reduction, consuming current which would otherwise deposit more metallic copper, or 2) reaction with previously deposited metallic copper, oxidizing and dissolving the copper. The net effect of either mechanism is reduced current efficiency. The second mechanism is more troublesome in that its pronounced occurrence at the solution level can result in failure of cathode hanger loops, i.e. cathodes falling to cell bottoms. The ferrous iron thus generated is re-oxidized to ferric at the anodes by 1) direct anodic oxidation or 2) reaction with the nascent oxygen produced there. The cycle repeats itself. To limit the ill effects, electrolyte iron content is commonly controlled at a manageable level of about 3 grams per liter by bleeding off spent electrolyte and replacing with sulfuric acid and water.
Spent electrolyte is typically maintained at about 30 grams copper per liter to provide quality cathode. Copper contained in electrolyte bleed is then ten times the iron content. If the copper/iron extraction ratio in SX is 100/l, then 10 percent of the total SX copper transfer is dedicated to electrolyte bleed.
This situation is generally tolerable in vat leach and agitated leach processes treating oxidized copper ores with net acid consumptions. The electrolyte bleed is input to the leach, the contained acid is utilized without affecting the acidity of the SX feed solution, and much of the contained copper is returned in the SX feed solution. On the other hand, some dump leaches and in-situ leach processes treating sulfide or mixed copper ores have no net acid consumption or are net acid producers. If electrolyte bleed is input to the leach the contained acid would not be utilized, but would be returned in SX feed to approximately the same extent as would the contained copper. This excess acidity would require substantially increased SX plant size and/or reagent concentration in the solvent to achieve the desired recovery. This means increased capital and/or operating costs, above and beyond those required to handle the new and recycled (electrolyte bleed) copper alone. Worse yet, the excess acidity in the leach system may destroy bacteria beneficial to the leaching. All of these problems would be exaggerated with a copper/iron extraction ratio of less than 100/l.
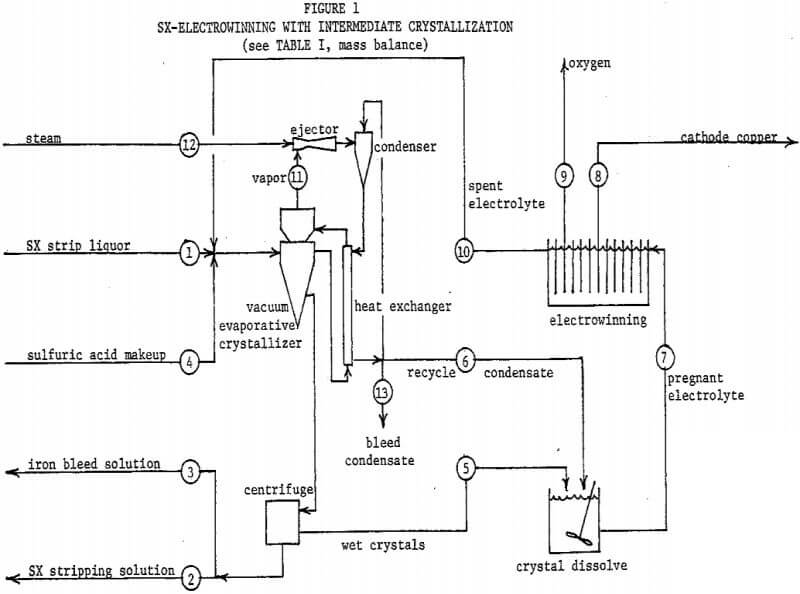
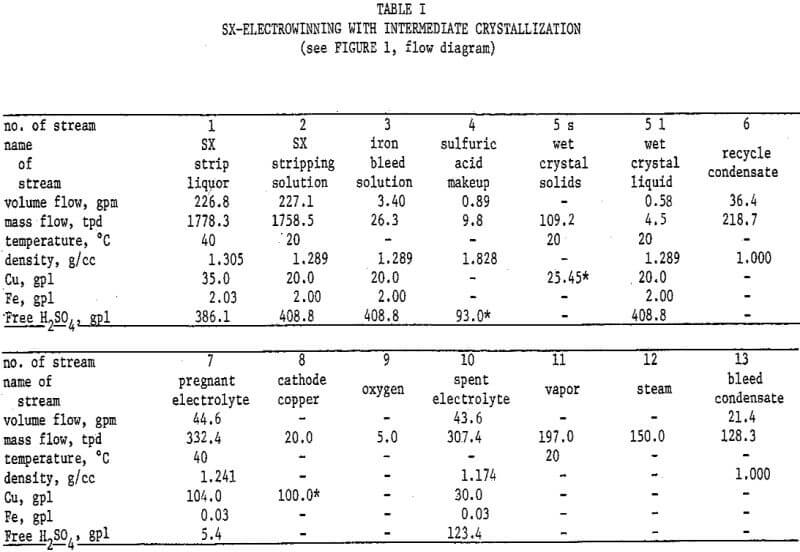
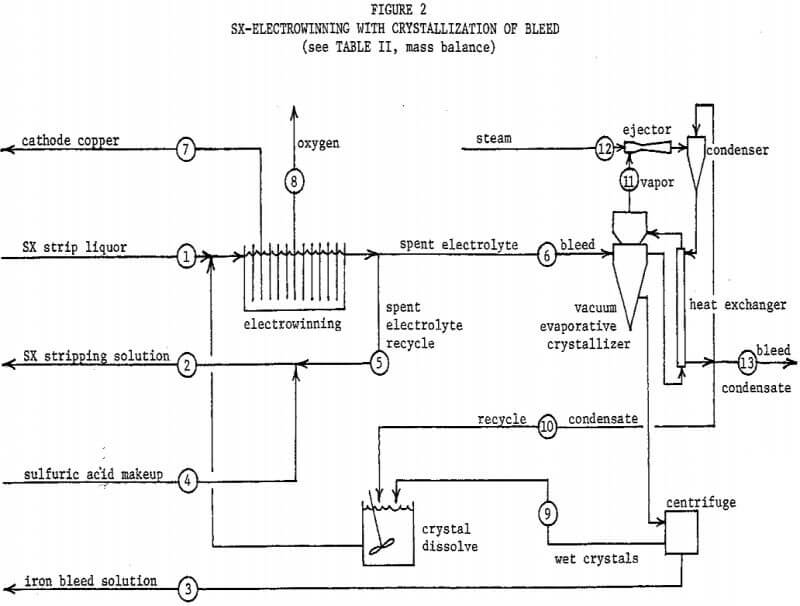
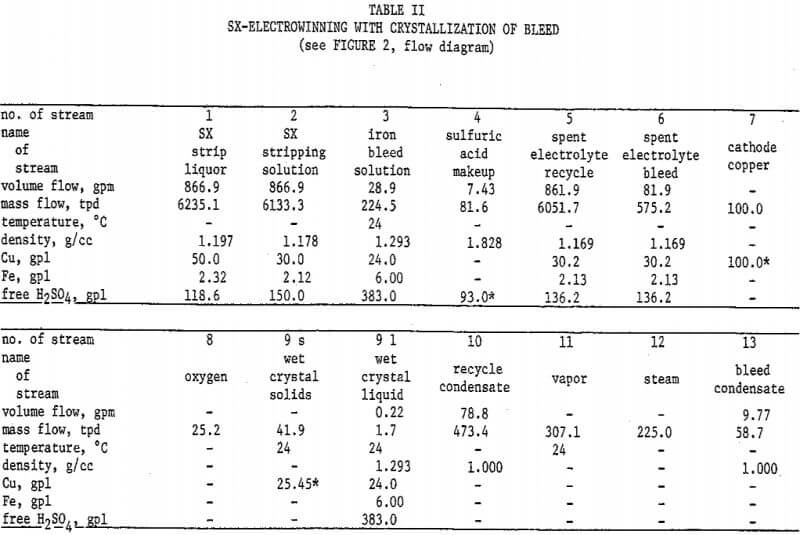
Figure 2 and Table II describe the possibility of utilizing copper sulfate crystallization to resolve these problems. The flowsheet basis is 100 tons cathode copper per day. The SX stripping solution and strip liquor compositions are appropriate for a circuit employing LIX 64N, and the copper/iron extraction ratio assumed is 100/l. A “throwaway” iron bleed solution is produced, containing only 4 percent of the total SX copper transfer. Toward justifying this loss, it must be pointed out that not all of the otherwise 10 percent copper bleed would be recovered if sent to the leach. An acceptable disposal method for this bleed solution would be required, such as an alkaline tailings pond from a sulfide flotation mill. It will be noted that spent electrolyte contains the desired 30 grams copper per liter, and as a bonus, substantially less than 3 grams iron per liter.
The crystallizer system, similar in all respects except sizing to that described for the first flowsheet, has been esimated at $700,000. Again assuming a steam cost of $5.25 per ton, the steam cost per pound of cathode copper is 0.59C.
The crystallizer temperature and pressure in this system would be 24°C and 15.6 mm Hg, respectively. The condenser would operate at 39 °C and 51 mm Hg. As explained for the first flowsheet, other cooling-heating systems might prove to be more economical.

