Table of Contents
In the past 25 years there has been a wide expansion of the use of cyanide heap leaching by the mining industry. This process allows profitable recovery of gold and silver from low-grade ore. Heap leach pads are constructed on slight drainage grades and lined with an impervious clay and/or geotextile. Ore is stacked and leached in successive lifts or benches on the pad. Sprinkler systems set within and on the surface of the lift to be leached deliver a high pH sodium cyanide solution that, in its percolation through the ore, forms stable complexes with gold, silver, cobalt, iron, copper, nickel, zinc, and cadmium. This pregnant solution is collected as it drains from the pad for precious metal recovery.
After leaching, the heap still contains interstitial and adsorbed cyanide species, including free cyanide and metal-complexed cyanides. The metallo-cyanides are present as weak acid dissociable (WAD) complexes of cadmium, copper, nickel, and zinc, and extremely stable iron and cobalt cyanide complexes (Smith and Mudder 1991). These cyanides have the potential to degrade surface and ground water resources.
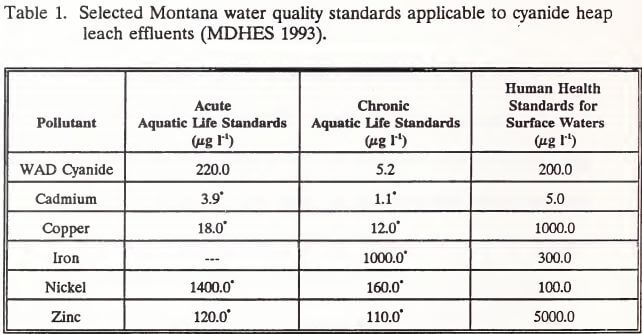
The Montana Water Quality Act directs the Montana Department of Health and Environmental Sciences (MDHES) to maintain standards for pollutants. The standards are referenced by the Montana Department of State Lands in mine operation and reclamation regulation. Standards of interest to cyanide heap leach mining are listed in Table 1.
Cyanide in the spent heap ore degrades naturally under the influence of natural climatic and biotic conditions if the heap is not disturbed. Natural degradation of cyanide can occur through volatilization, biodegradation, photodecomposition, and oxidation (Rolfes 1989). Other degradation mechanisms associated with cyanide chemistry include complexation with transition metals, precipitation of complex cyanide compounds, formation of thiocyanate, and the hydrolysis of free cyanide (Smith and Mudder 1991). How degradation will proceed, though, is site specific, depending on the interaction between geochemical conditions in the heap and site conditions such as precipitation, ore mineralogy, and ore permeability (Struhsacker and Smith 1990, van Zyl et al. 1988). To speed this degradation process, most mines use fresh or chemically-treated water rinses. Rinsing is continued until effluent draining from the pad falls and remains below a regulatory standard for a specified period of time. Currently, the Montana Department of State Lands considers a heap to be neutralized for reclamation when effluent WAD cyanide concentrations remain below 0.220 mg l-1 through one full spring after rinsing. This WAD cyanide standard, commonly used by western state mine regulators, is 20 µg l-1 above the MDHES human health standard (Table 1).
Despite acceptable effluent levels from abandoned heaps, later “spikes” or “slugs” of cyanide have been measured in effluent following large precipitation events or rinsing (Haight 1991, Struhsacker and Smith 1990). It is believed that residual cyanide in spent ore, pore spaces, and/or blind-off zones diffuses out into the heap over time. Subsequent wetting fronts generated by precipitation events may pick up this cyanide as they pass through the heap, degrading effluent water quality. Spent ore sampling that does not examine the full depth and breadth of a heap may not give a representative measure of neutralization success.
The concentration of cyanide throughout a decommissioned heap has not been thoroughly investigated. With funding supplied by the Montana Department of Natural Resources and Conservation and the cooperation of the Montana Department of State Lands, Hardrock Bureau and Canyon Resources Corporation, the Reclamation Research Unit of Montana State University sampled a decommissioned heap from October, 1991 through summer 1992. The objective of this study was to measure the concentration of residual cyanide species and associated metals throughout the heap, comparing the results for evidence of vertical or areal distribution patterns. A second objective was to monitor cyanide and metals levels in pore water at the base of the heap.
This study was limited to one relatively small heap with limited construction and operation documentation. No control data were available for ore leached in the heap. The study was not a definitive work explaining the behavior of residual cyanide in neutralized heaps. Rather, it will provide basic data to decision makers regarding the concentrations and distributions of cyanide species that may develop under similar conditions.
History of the Cyanide Leach Process
The evolution of cyanidation technology in the pursuit of precious metals dates back 200 years. In 1793, potassium cyanide was recognized as a gold solvent (Hiskey 1985). In 1844, Elsner investigated the reactions of various metals in a potassium cyanide solution, discovering that gold dissolution took place only in the presence of dissolved oxygen (Bosqui 1899). This dissolution reaction, known as Elsner’s Equation, is written as:
4 Au + 8 KCN + O2 +2H2O = 4 AuK(CN)2 + 4 KOH
In 1896, Bodlaender found that the dissolution of gold was a two-step reaction with intermediate production of hydrogen peroxide (Hiskey 1985). This reaction proceeds as follows:
2 Au + 4 CN + O2 + 2 H2O = 2 Au(CN)2 + 2 OH + H2O2
2 Au + 4 CN + H2O2 = 2 Au(CN)2 + 2 OH
The sum of this reaction sequence is equivalent to the dissolution reaction developed by Elsner. It is written as:
4 Au + 8 CN + O2 + 2 H2O = 4 Au(CN)2 + 4 OH
The extraction of gold from gold ore using cyanidation was developed into a commercial process in Scotland by John MacArthur and Drs. Robert and William Forrest (von Michaelis 1985). They were issued patents for their process in 1887 (Britain) and in 1889 (United States). The extraction is based on the agitation of finely ground gold ore in aerated potassium cyanide solution. Metals in the ore, including gold, complex with cyanide during the agitation. The gold cyanide complex is precipitated with zinc shavings (Eisele 1988). This precipitation proceeds as follows:
2 Au(CN)2 + Zn = 2 Au + Zn(CN)4²
The Merrill-Crowe process, as it is known in use today, improved gold recovery by removing oxygen from gold-bearing cyanide solutions before the addition of zinc (Hiskey 1985). After treatment with zinc, the solution is filtered under pressure to remove the gold precipitate for smelting.
Carbon adsorption technology to recover dissolved gold from cyanide solutions was developed by Chapman in 1939 (Hiskey 1985). This technology did not require the filtration and deaeration of gold-bearing solutions as did the Merrill-Crowe process (Heinen et al. 1978). Activated charcoal, introduced to a cyanide-ore pulp (slurry), adsorbed the dissolved gold and floated away for collection (Hiskey 1985).
Heap Cyanidation
Heap leaching may be defined as the percolation leaching of piles of low-grade ores that have been stacked or piled on specially prepared watertight drainage pads (Heinen et al. 1978). Heap leaching of minerals dates back to sixteenth century Hungarian copper mines, with large-scale leaching being developed by the eighteenth century in Spanish copper mines (Hiskey 1985). Uranium producers have utilized heap leaching as an extraction technology for low-grade ores since the late 1950s (Dorey et al. 1988). Heap leach cyanidation of porous, low-grade, gold-bearing ores was first proposed in 1967 by U.S. Bureau of Mines metallurgists (Heinen et al. 1978). This treatment chemistry is the same as that of Bodlaender’s two-step dissolution reaction. Gold is recovered from the pregnant solution draining from the heap using either Merrill-Crowe or carbon adsorption processes.
In order to widen the applicability of cyanide heap leaching to include impermeable high-clay ores, the U.S. Bureau of Mines developed an ore agglomeration treatment. After crushing, ore is mixed with 2 to 7 kg/metric ton (5 to 15 lbs/short ton) of portland cement (to act as a binding and pH control agent), wetted with 8 to 16% (by weight) water, and subjected to mechanical tumbling to ensure adhesion of fines to coarser particles (Heinen et al. 1978, Heinen et al. 1979, McClelland and Eisele 1982). By 1983, it was estimated that agglomeration pretreatment allowed half of cyanide heap leach projects to operate (McClelland et al. 1983).
The cyanide heap leach process is an efficient, low cost method of recovering gold and silver from low-grade ore. Average ore grades recovered by heap leaching are 0.9 grams of gold per metric ton of ore (0.03 oz/short ton), with an extreme cutoff grade of 0.2 grams of gold per metric ton (0.006 oz/short ton) at the Round Mountain Mine in central Nevada (Spickelmier 1993). Relative ore grade dictates the choice of two heap leaching methodologies. Higher grade ores are crushed, agglomerated and stacked in short lifts of 1 to 3 m (3 to 10 ft) high on permanent asphalt or concrete leach pads. This method treats 900 to 9,000 metric tons (1,000 to 10,000 short tons) of ore for 7 to 30 days, after which the spent ore is neutralized and removed for disposal (Higgs and Gormley 1992a, Rolfes 1989). Lower grade ores are typically not treated, being stacked as run-of-mine size (including boulders) in lifts of 3 to 9 m (10 to 30 ft) high on compacted clay and/or synthetic-lined pads. On these heaps, leaching of 9,000 to 1.8 million metric tons (10,000 to 2 million short tons) of ore will take months or years, after which the spent ore heap is neutralized and abandoned (Hiskey 1985, Rolfes 1989).
Pads under all heaps are constructed with a 2 to 5% slope to facilitate drainage of pregnant solution (Chamberlin and Pojar 1984). Pads are also designed to minimize the loss of solution to the underlying strata and prevent contamination of local water sources (Heinen et al. 1978).
The objective of heap leach construction is to build a stable, porous heap that allows an even downward percolation of cyanide solution (Herzog 1990).
Heaps are commonly built in successive lifts using conveyor stacking, end dumping, or truck and dozer techniques. The criteria in selecting a heap construction technique are to minimize layering, compaction, and ore particle segregation (Dorey et al. 1988).
The completed heap is put under cyanide leaching by a surface and/or subsurface network of sprinklers or capillary tubes. Typical application rates, adjusted to result in a partially-saturated flow of leachate through the heap, range from 0.002 to 0.003 1/s/m² (0.003 to 0.005 g/min/ft²) (Dorey et al. 1988). Though it is possible to leach gold with several types of solutions, all current commercial operations use 0.2 to 0.7 kilograms of sodium cyanide (NaCN) per metric ton of water (0.5 to 1.5 lbs of NaCN per short ton of water) (Chamberlin and Pojar 1984, Stanton et al. 1986). This produces a leach solution concentration of 200 to 700 mg l-1 NaCN, or 125 to 350 mg l-1 free cyanide (Stanton et al. 1986). Leach solutions are maintained at pH 9.5 to 11 with lime (CaO) or caustic soda (NaOH) to ensure effective gold dissolution (Chamberlin and Pojar 1984). Leaching consumes NaCN at a rate of 0.04 to 1.4 kg/metric ton (0.1 to 3.0 lbs/short ton) of ore, depending on ore type, application method, porosity, solution pH, and the concentration of cyanide-consuming metals and sulfides in the ore (U.S. EPA 1986, Stanton et al. 1986).
Heap Neutralization
The consumption of NaCN is a major expense in heap leach operations that exploit low-grade ore (Barratt and McElroy 1990). To recover NaCN for use on other leach heaps and maximize precious metal recovery, leached ore is thoroughly rinsed with fresh water at the end of a leaching cycle (Heinen et al. 1978). This recovery action can be regarded as the initiation of heap neutralization. The ultimate purpose of this washing or rinsing is to alter the chemistry of the reclaimed heap so that future drainage from the heap degrades neither surface or groundwater resources (Struhsacker and Smith 1990). Effective neutralization of spent ore heaps involves detoxifying residual cyanides and cyanide complexes found in interstitial solutions, adsorbed on ore particle surfaces, and diffused into the ore (Denton et al. 1992).
Active neutralization methodologies in use today include fresh water rinsing, alkaline chlorination, hydrogen peroxide treatment, sulfur dioxide oxidation, biotreatment and acidification. These methods are well documented by Scott (1984), Rolfes (1989), and Thompson and Gerteis (1990).
Cyanide In The Neutralized Heap
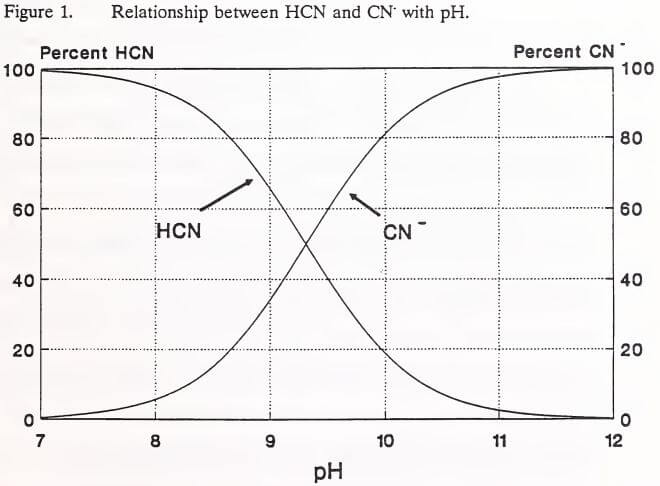
Cyanides comprise a large class of compounds that are characterized by the presence of the cyanide ion (CN-) in their molecular structures (Higgs and Gormley 1992b). The forms of cyanide of particular interest to the heap leach process are free cyanide, simple cyanide and complex cyanide (U.S. EPA 1986). Free cyanide is the sum of the cyanide ion (CN-) and hydrocyanic acid (HCN) species released into an aqueous solution by the dissolution and dissociation of cyanide compounds (Mudder 1991). These two species coexist in solution, their relative proportions depending on pH and temperature. The relationship between solution pH and the ratio of CN- to HCN is illustrated in Figure 1. Below a pH of 7.0, all free cyanide is present as HCN in the toxic gaseous state.
Simple cyanides are the salts of hydrocyanic acid that completely and easily dissociate in water (Maynard et al. 1986). These cyanides ionize in water, as sodium does in Reaction [1].
NaCN = Na+ + CN……………………………………..[1]
The cyanide ion (CN ) then hydrolyzes to form HCN in Reaction [2],
CN + HOH = HCN + OH…………………………….[2]
Again, the amount of HCN produced depends on solution pH and temperature.
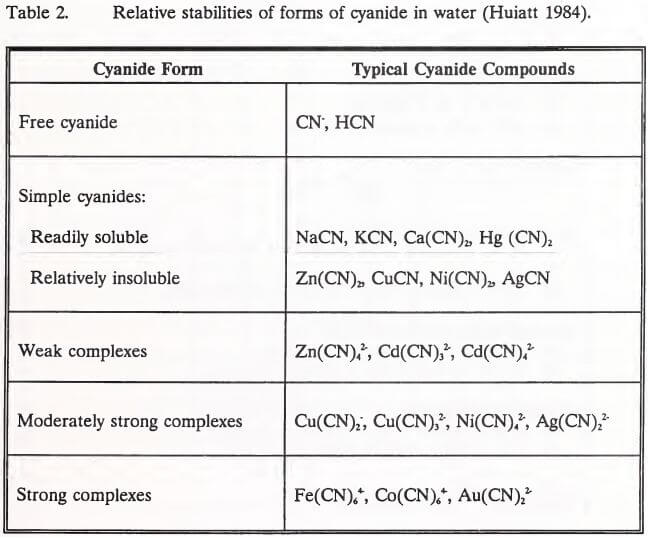
In the heap environment, the formation of gold and silver cyanide complexes is the means of precious metal extraction from ores. Other transition metals present in the ore may also complex with cyanide. Most commonly, these metals are cadmium, copper, cobalt, iron, nickel and zinc (IEC 1979). The stability of these metal-cyanide complexes depends on the stability of the particular metal. Cadmium and zinc are relatively unstable, easily dissociating to release the cyanide ion (CN). The dissociation of complex cyanides is inversely related to solution pH and complex ion concentration (Huiatt et al. 1983). The toxicity of complex cyanides is related to their ability to release cyanide ions to solution, producing toxic HCN (Eisler 1991). Metals freed by the dissociation of complex cyanides in a heap are not considered a threat to effluent water quality. Solubility of metals is limited at the high pH levels (7.5 to 9.5) normally observed in leach heaps rinsed of cyanide (Lindsay 1979). Further, once a complex cyanide degrades, the metal would be expected to precipitate as a hydroxide or to adsorb onto solids surfaces (Schafer et al. 1991). The relative stabilities of the forms of cyanide common in leach heap solutions are shown in increasing order of stability in Table 2 (Huiatt 1984).
Regardless of the method of heap neutralization, natural degradation of residual cyanide and metal-cyanide complexes will occur after abandonment. Smith and Struhsacker (1988) have detailed a schematic showing potential geochemical conditions and cyanide reactions in and around an abandoned heap.
Table 3 lists the chemical reactions and their equations, which are noted in schematic form in Figure 2.

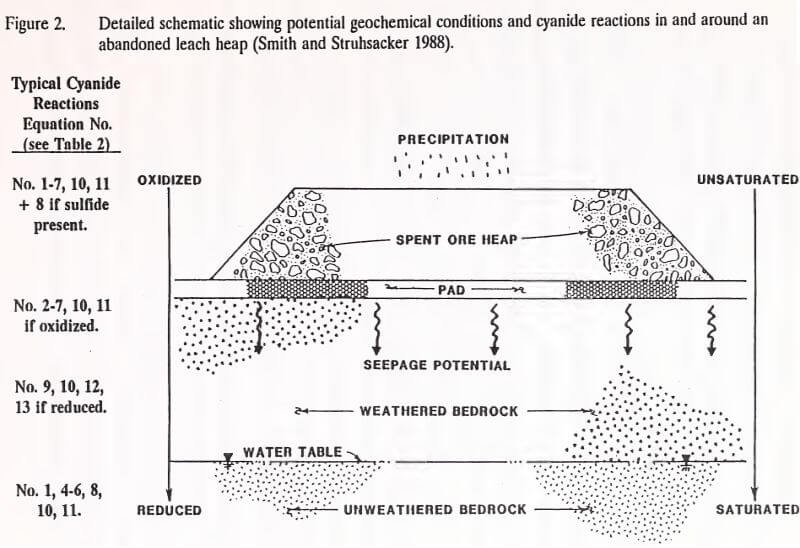
The degree and rate of natural cyanide degradation in the heap is a function of the type of cyanide species present, their concentration, ore mineralogy, available bacteria, heap permeability, and site conditions (temperature, precipitation, elevation) (Denton et al. 1992). Free cyanide in the heap degrades quicker than simple and complex cyanides. Cyanide reactions consuming free cyanide include hydrolysis and volatilization with decreasing pH, oxidation, hydrolysis/saponification, biodegradation, and thiocyanate formation (Smith and Struhsacker 1988). The mineralogy of ore in the heap determines the types and amounts of metals available for metal-cyanide complexation and sulfides for the formation of thiocyanate. Biodegradation of free cyanide requires bacteria and an energy source. A permeable heap ensures an oxygen supply for oxidation and aerobic biodegradation reactions. It also ensures a water supply for hydrolysis, hydrolysis/saponification, and solute transport processes (Smith and Struhsacker 1988). Increases in temperature will speed reaction rates. Higher elevation locations will experience increased volatilization of HCN from the aqueous phase.
Mine operators are required by regulators to neutralize spent heaps to protect human health and the environment. Neutralization criteria vary from state to state, requiring analysis of heap effluent or leachate extracted from heap ore (Heriba 1991). The establishment of effective neutralization criteria is hampered by a confusion of terminology used to describe cyanide forms and the variety of analytical methods used to measure them (Smith and Struhsacker 1988).
Huiatt (1984) classified cyanide and cyanide compounds into five forms according to their stability (Table 2). Over the past decade, however, three analytical terms (total, WAD, and free cyanide) have come into common use in describing cyanide species. These terms and the cyanide compounds they represent are listed in Table 4.
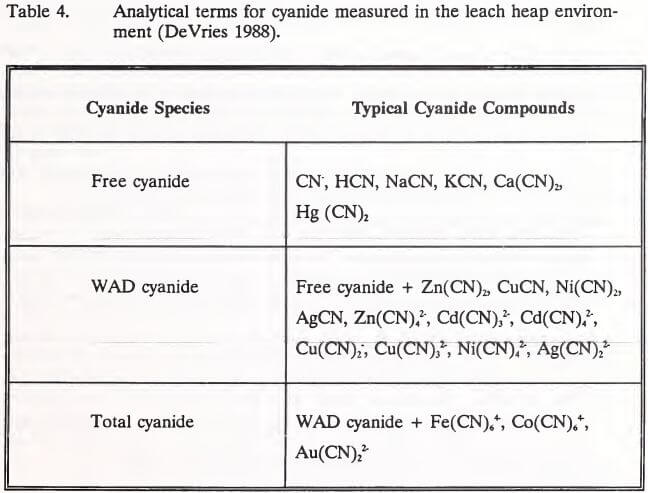
Free Cyanide Measurement
The objective of free cyanide analysis is to measure the amount of CN and HCN in a given sample (Maynard and Szczahor 1992). This measure will also include the readily soluble simple cyanides (Maynard et al. 1986). American Society for Testing and Materials (ASTM) Procedure D4282 (1993) analyzes HCN which diffuses from a sample solution chamber (buffered at pH 6) to a NaOH solution chamber in four hours. Colorimetric analysis of the diffused solution is sensitive from 0.010 to 0.150 mg l-1 HCN. Diffusion methods for HCN in mine wastes suffer interference from the production of HCN by metal-cyanide complex dissociation (Maynard and Szczachor 1992). The American Public Health Association (APHA) Cyanide-Selective Electrode Method 4500-CN-F (1992) measures CN- concentrations in a range of 0.05 to 10 mg l-1 in sample solutions. Cyanide ion-selective electrode readings are compared to a cyanide standard calibration curve and a CN- concentration is calculated. This method is particularly sensitive to interference from the precipitation of sulfides, chlorides, and mercury on the cyanide ion-selective electrode (Smith and Struhsacker 1988).
WAD Cyanide Measurement
The measurement of WAD cyanide in effluent solutions is most commonly performed using reflux distillation in APHA Standard Method 4500-CN-I (1992) or ASTM D-2036 Test Method C (1993). Distillation of sample solution (buffered at pH 4.5) for one hour liberates HCN from free cyanide and weak metal-cyanide complexes. Collected in a NaOH absorption solution, WAD cyanide is best estimated using direct colorimetric determination, accurate to 0.03 mg l-1 (ASTM D-2036 Test Method C 1993). The reflux distillation method recovers all the cyanide from complexation with zinc and nickel, 70 percent from copper complexes and 30 percent from cadmium complexes (Smith and Struhsacker 1988). Cyanide is not recovered from its strongest complexes with iron, cobalt or gold. The appeal of this method is that it is relatively free of thiocyanate or sulfide interference, and other interferences can be removed before distillation (Mudder 1991).
A less rigorous, less accurate method of measuring WAD cyanide concentrations in mine effluents is the picric acid method (Smith and Mudder 1991). This method, accurate to 0.5 mg l-1, involves the development of color with picric acid in the presence of nickel while heating over a water bath for 20 minutes, followed by colorimetric measurement (Smith and Mudder 1991). Though less accurate, it can be used more easily for on-site monitoring of effluents for WAD cyanide concentration changes (Maynard and Szczahor 1992).
A new method for measuring WAD cyanide is found in Kelada’s procedure for cyanide speciation (Kelada et al. 1992). It was adopted by ASTM as Automated Methods for Determination of Total Cyanide, Dissociable Cyanide, and Thiocyanate (D4374) in September 1991 (ASTM 1993). This procedure uses acidification and thin film distillation to free cyanide from weak metal complexes, capturing HCN in a NaOH absorber for colorimetric measurement. It is free of thiocyanate interference and results are regarded as more reliable than distillation methods (Kelada et al. 1992).
Total Cyanide Measurement
Analytical methods to measure total cyanide in wastewater can use manual or automated procedures. The most widely accepted procedure for determining total cyanide in mine effluents is the manual reflux distillation in APHA Standard Method 4500-CN-C (1992), ASTM D-2036 Test Method A (1993), or EPA 9010/ 9012 (1986). This procedure is almost the same as that used in reflux distillation for WAD cyanide measurement. However, the total cyanide reflux distillation procedure uses a strong acid (1:1 sulfuric acid) to free cyanide from stronger iron complexes. Interference may be caused by the presence of thiocyanate and sulfide (Smith and Mudder 1991).
The ASTM D4374 (1993) Automated Method for Determination of Total Cyanide, Dissociable Cyanide, and Thiocyanate uses ultraviolet radiation to free cyanide from the strongest iron, cobalt, gold and platinum complexes. As in the measurement of WAD cyanide, Kelada (1992) reports this method to be free from thiocyanate interference and regards it as more reliable than distillation methods in the measurement of total cyanide concentrations.
Measurement of Cyanide in Solids
Cyanides can also exist on and in spent heap ores. Their measurement in the past has typically involved homogenization and direct distillation of a solid sample or leachate using ASTM D-2036 (1993) or EPA 9010 (1986) methods (Hendrix et al. 1985, Durkin 1990, and Davis et al. 1991). Most recently, an ongoing Bureau of Mines cyanide degradation study (Comba et al. 1992) adopted a sample preparation procedure whereby frozen ore is crushed to 10 to 20 mesh size. Subsequent cyanide analysis uses 110 to 120 grams of the frozen sample in direct distillation using ASTM D-2036 (1993) procedures. A study to develop a standard solid sample analytical procedure (Davis et al. 1991) concluded that 60-day bottle-roll extraction using 1.25 N NaOH solution, followed by leachate distillation, provided the most reliable WAD and total cyanide measurement. The study recommended further refinement of bottle roll extraction methods.
Residual Cyanide Studies
As more and more heap leach projects undertaken in the past 25 years near completion, effective neutralization of residual cyanide is of interest to both the mineral industry and regulators. Little information is available on residual cyanide levels following heap neutralization from the few projects decommissioned to date. Engelhardt (1985) found that approximately 85% of the free cyanide present in an untreated, inactive silver leach heap in California was degraded by natural processes over an 18 month period. The pH of the agglomerated ore declined from 10.5 to 9 through the course of this study. The Golden Maple-Gilt Edge gold heap leach project near Lewistown, Montana, was closed by regulators in late 1985 (Schafer et al. 1991). Leached ore had a high clay content but was not agglomerated. The heap was rinsed with calcium hypochlorite after closure. Subsequent inspection of the heap as it was excavated showed zones of high residual cyanide that were not effectively rinsed.
The Borealis Mine in Hawthorne, Nevada was deactivated in 1987 after two year’s cyanide leaching of agglomerated ore (Schafer et al. 1991). Twenty-two months later, the heap was rinsed with fresh water to reduce effluent WAD cyanide concentrations to less than 0.2 mg l-1. For five months following this neutralization, effluent WAD cyanide levels remained less than 0.2 mg l-1 and the pH remained below 8.5 in most samples. Ore sampled from three backhoe pits had total cyanide levels below the 10 mg kg¹ limit set by the Nevada Division of Environmental Protection.
In their study of the Gilt Edge Mine heap leach operation in Deadwood, South Dakota, Smith and Mudder (1990) concluded that sampling of ore is a superior method of assessing neutralization success compared to heap effluent monitoring. In a study of neutralization at Kendall Mine’s Heap Leach Pad No. 1 and Pad No. 2, Haight (1991) found total cyanide levels increasing from the surface to 6 m (20 ft) depth. He concluded that spent ore must be sampled at various depths and locations throughout a heap, as surface ore or effluent sampling will not accurately characterize the cyanide concentrations within it.
Schafer et al. (1991) conducted an intensive investigation of the behavior of cyanide and metals in a spent leach heap at the Landusky Mine near Malta, Montana. Ore in the heap was very permeable and was not agglomerated. Heap excavation and neutron probe data showed no evidence of blind-off zone development. Natural degradation processes were predicted to reduce WAD cyanide levels to less than 0.22 mg l-1 within 6 to 8 years. The addition of one pore volume of fresh water rinse reduced cyanide, nitrate and metal levels by 50 to 90 percent. Soluble metal levels were found to be closely related to the level of cyanide in solution. WAD cyanide levels less than 0.22 mg l-1 in heap effluent had copper levels 4%, zinc levels 1%, iron levels 47%, and cadmium levels less than 10% of the drinking water standard for each metal (Schafer et al. 1991).
The Snow Caps Mine gold leach heap near Independence, California was neutralized in 1991 using the INCO SO2/Air process. Subsequent auger drill sampling found total cyanide levels below the regulatory level of 10 mg kg¹ in 9 of 10 samples (Young et al. 1992). In an ongoing study of natural degradation of cyanide forms in an inactive spent heap at the Trinity Silver Mine in Nevada, preliminary data indicate total and WAD cyanide levels are higher at lower heap depths (Comba et al. 1992). Natural cyanide degradation rates are slow, but are expected to increase as leach solution more completely drains from the heap.
Study Area
The Kendall Mine is located in the North Moccasin Mountains, approximately 32 kilometers (20 miles) northwest of Lewistown, Montana (Figure 3). Gold has been produced in this area since the late 1800s advent of cyanide
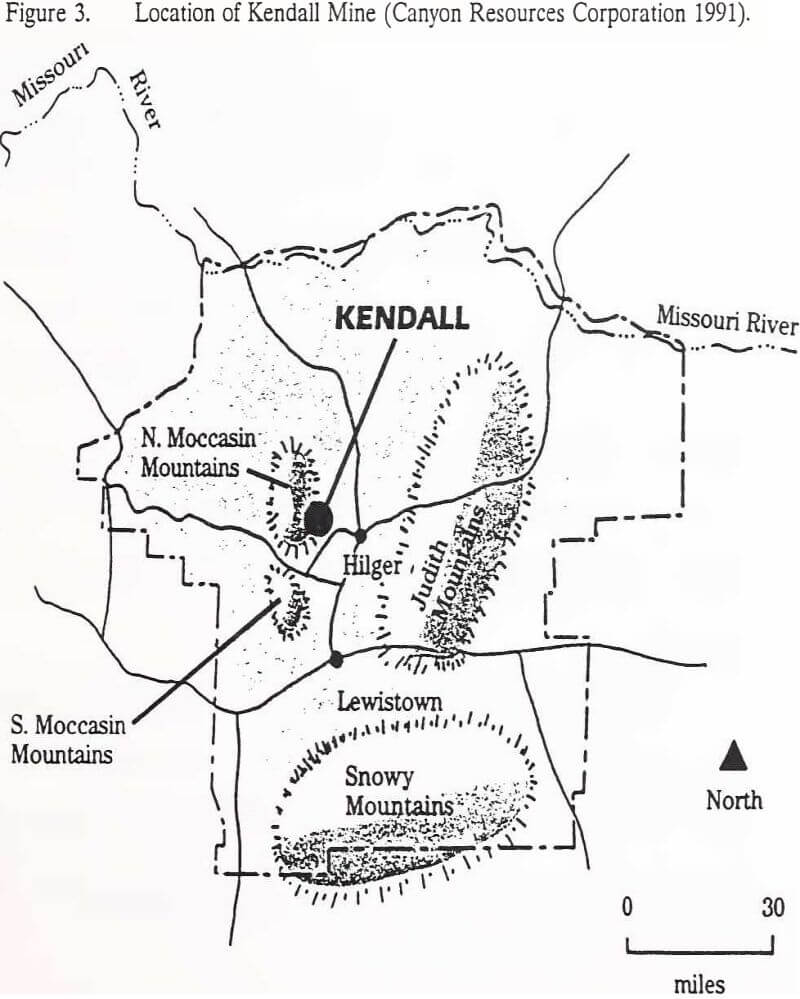
vat leaching. Open pit heap leach mining of gold began in 1982. After several ownership transfers, Canyon Resources Corporation acquired Kendall Mine in February 1988. The mine project is currently permitted for operation in Township 18N, Range 18E, Sections 29, 30, 31 and 32 and Township 17N, Range 18E, Section 6. Heap Leach Pad No. 1, the focus of this investigation, is located in Township 18N, Range 18E, Section 31.
Construction of Heap Leach Pad No. 1 was not thoroughly documented. The heap covers 1 hectare (2.4 acres) and contains 82,000 metric tons (90,000 short tons) of agglomerated, run of mine ore on a compacted clay liner (Haight 1991). The heap was constructed in two lifts using either end dump or truck and dozer techniques. The sandy gravel ore at the top of Pad No. 1 is comprised of 62% gravel, 24% sand, and 14% clay (HKM Associates 1982). In August of 1988, the new owners ripped the surface of the heap to increase permeability and subjected it to cyanide leaching during the next month. The heap was leached for 68 hours with approximately 760,000 liters (200,000 gallons) of sodium cyanide solution. Insufficient gold was found and the heap was left to drain before spring decommissioning.
Active neutralization of Heap Leach Pad No. 1 began in March, 1989 with one week of fresh water rinsing. Prior to chemical neutralization, ore at the surface was sampled for cyanide. Results are shown in Table 5. Effluent discharge from the heap at this time measured 49.0 mg l-1 total cyanide and had a pH of 8.6.
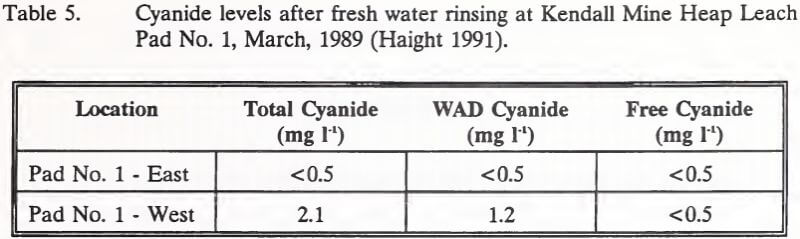
Neutralization of the heap with calcium hypochlorite followed the rinsing and continued through May, 1989. At this point, effluent draining the heap measured 0.1 mg l-1 total cyanide with a pH of 8.8. By the end of September, 1989, effluent draining the heap measured 0.02 mg l-1 total cyanide with a pH of 8.1.
Methods
Sampling Methods
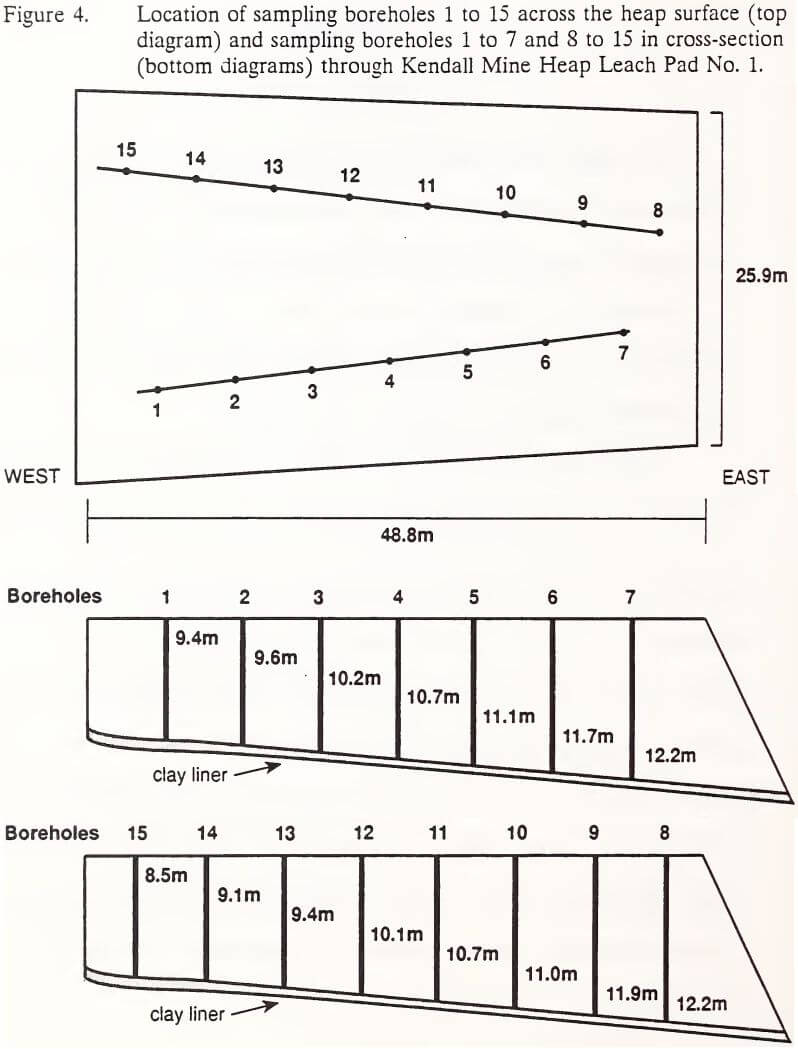
Ore samples were collected from Kendall Mine Heap Leach Pad No. 1 from October 29 to November 2, 1991, thirty months after active chemical neutralization ceased. Fifteen boreholes were located along two random transects across the heap in a randomized complete block field plot design (Figure 4). Boreholes were regarded as blocks and sampled depth increments as treatments. Block/depth interaction was assumed to be negligible.
Samples were taken from nine to eleven depth increments: 0 to 0.2, 0.2 to 0.5, 0.6 to 1.2, 1.5 to 2.1, 3.0 to 3.7, 4.6 to 5.2, 6.1 to 6.7, 7.6 to 8.2, 9.1 to 9.8, 10.7 to 11.3, and 12.2 to 12.8 m (0 to 0.5, 0.5 to 1.5, 2 to 4, 5 to 7, 10 to 12, 15 to 17, 20 to 22, 25 to 27, 30 to 32, 35 to 37, and 40 to 42 ft), with incremental sampling halted at the clay liner. A truck-mounted drill rig using a hollow stem auger was used to bore into the heap, pausing at the top of each sampling depth to pound a split spoon sampler through the depth increment. Retrieved split spoon samples were halved along the length of the spoon and examined for lithological characterization. One half of the sample was bagged for cyanide analyses. The other half was bagged for metals analyses. Both samples were sealed in air-tight plastic bags, logged and stored in coolers. Sample splits for cyanide analyses were shipped to Energy Laboratories in Billings, Montana for measurement of total, WAD, and free cyanide.
Split spoons were decontaminated between samples with a wire brush and cloth wipe. Auger sections were decontaminated between boreholes by pressurized steam cleaning. Any contact with the clay liner was backfilled with bentonite pellets. Boreholes were backfilled with drill cuttings after sampling was complete.
Three boreholes were fitted with pore water samplers (suction lysimeters) prior to backfilling (Table 6). These three suction lysimeters were installed to monitor pore water quality near the heap base. The suction lysimeter installed in Borehole 13 was accidentally destroyed by a mine grader before the sampling program commenced.
Pore water samples for cyanide analyses were collected 6 to 8 hours after negatively pressurizing the suction lysimeters. These samples were collected directly into opaque sample bottles. They were preserved for cyanide analyses by the addition of sodium hydroxide to pH 12 and cold storage. After this sampling, the suction lysimeters were again negatively pressurized for collection of additional pore water samples 12 hours later. The second set of samples was used for metals analyses. Upon collection, these samples were measured for pH. Samples were then preserved for metals analyses by the addition of nitric acid to pH 2 and cold storage.
Analytical Methods
Because standard methods for a solids cyanide analysis applicable to mine ore were, as they remain, in a state of flux (Comba et al. 1992, Smith and Mudder 1991), ASTM (1993) D-2036 Test Method C reflux distillation procedures for cyanide in liquids were modified to measure spent ore total and WAD cyanide levels. The modification consisted of adding one-half gram of <2 mm air-dried ore sample to the reflux distillation solution prior to analysis. Free cyanide in the ore solutions was measured using a cyanide ion-specific electrode (APHA Method 4500-CN-F 1992).
Saturated paste extracts of oven-dried ore samples were prepared for pH and soluble metals measurement using American Society of Agronomy Method 10-2.3.1 (Rhoades 1982). Soluble cadmium, copper, iron, nickel, and zinc levels were measured in the saturated paste extracts by atomic absorption spectroscopy.
Pore water samples were analyzed for total and WAD cyanide using ASTM D-2306 Test Method C (1993). Free cyanide was measured using APHA Method 4500-CN-F (1992). Atomic absorption spectroscopy was used to measure soluble cadmium, copper, iron, nickel and zinc concentrations in pore water samples.
To monitor the precision of analyses, blind field replicates and laboratory replicates were entered into the sample set at a 5% (1 in 20) rate. Blind field replicate relative percent difference (RPD) averaged 18.4% total cyanide, 16.0% WAD cyanide, 3.1% pH, 5.6% EC, 19.1% cadmium, 23.4% copper, 22.8% iron, 9.3% nickel, and 30.9% zinc. Laboratory replicate RPD averaged 23.2% total cyanide, 10.8% WAD cyanide, 1.6% pH, 3.9% EC, 11.8% cadmium, 21.9% copper, 6.2% iron, 20.1% nickel, and 19.5% zinc.
Laboratory natural matrix spikes and laboratory digestion/extraction spikes were utilized at a 5% rate to monitor the accuracy of cyanide analyses. Natural matrix spike recoveries for total and WAD cyanide averaged 83.0% and 58.9%, respectively. Laboratory digestion/extraction spike recoveries for total and WAD cyanide averaged 98.4% and 96.1%, respectively. Accuracy of pH and metals analyses were monitored using laboratory standards at a 10% (1 in 10) rate. Recoveries averaged 100.3% pH, 96.1% EC, 98.1% cadmium, 97.6% copper, 98.8% iron, 101.1% nickel, and 99.3% zinc.
To clarify interpretation, the most complete data (from the upper nine of the eleven depth increments sampled for all boreholes) were combined into five depth categories. Measurements below analytical detection limit were adjusted for inclusion in the development of the five depth categories. This adjustment multiplied respective analytical detection limits by a factor of 0.7 to obtain a numerical value (Severson 1979). The five depth categories were 0 to 0.5, 0.6 to 2.1, 3.0 to 5.2, 6.1 to 8.2, and 9.1 to 9.8 m (0 to 1.5, 2 to 7, 10 to 17, 20 to 27, and 30 to 32 ft).
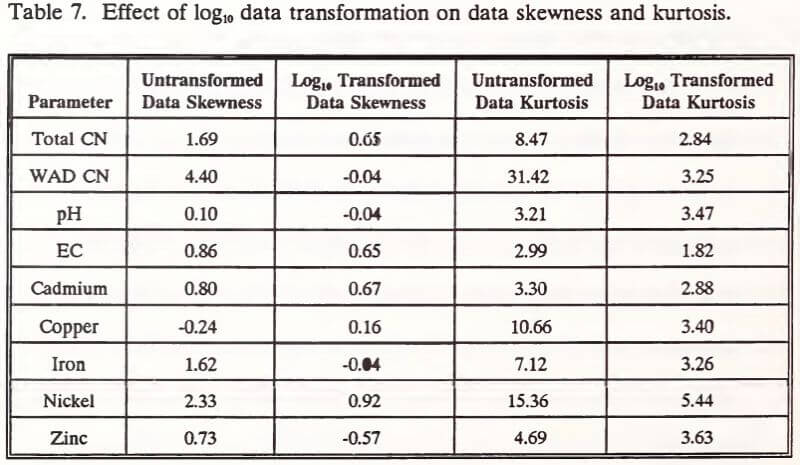
Skewness and kurtosis of untransformed data from the five depth categories (Table 7) indicate nonnormality in six of eight parameters at the 90% confidence level. With a sample size of 75, skewness should lie within the range of -0.34 to +0.34 to indicate a normal data distribution at the 90% confidence level (Sachs 1982). Likewise, kurtosis should lie within the range of 2.40 to 3.50 (Sachs 1982). These data were normalized by a log10 transformation. The transformation did not improve normality in four of nine parameters (Table 7). Transformed EC (skewness and kurtosis), cadmium (skewness), nickel (skewness and kurtosis), and zinc (skewness and kurtosis) data were not within the ranges reported by Sachs (1982). Despite indications of nonnormality, transformed EC, cadmium, nickel, and zinc data were included in subsequent statistical analysis and interpretation.
Overall two way analysis of variance and its source components (depth and borehole) for normalized total cyanide, WAD cyanide, pH, EC, and metals data were evaluated at the 95% confidence interval using the PROC GLM procedure in SAS (SAS Institute 1987). Where the p-value for the observed F-statistic was less than or equal to 0.05, the hypothesis of equality of means was rejected. Data sets with unequal means were then subjected to the Student-Newman-Keuls means separation procedure at the 0.05 level of significance.
Pore water data collected from suction lysimeters in boreholes 4 and 8 were subjected to linear regression at the 95% confidence interval. This regression was performed in order to identify trends in cyanide and metals levels in pore water.
Results and Discussion
All solids analytical data are found in Appendix A. Data validation tables are found in Appendix B. Statistical output from ANOVA, means separations, and linear regressions are provided in Appendix C. Data presented in this section are summaries of this information.
Many spent ore sample analyses showed cyanide at or below detection limits (Appendix A, Tables 12, 13, and 14). Samples which had total cyanide measurements below the analytical detection limit were not further analyzed for WAD or free cyanide. Similarly, samples which revealed WAD cyanide concentrations below analytical detection limits were not further analyzed for free cyanide. With two exceptions, free cyanide was either below analytical detection limits or not analyzed.
Cyanide and Metals Depth Distributions
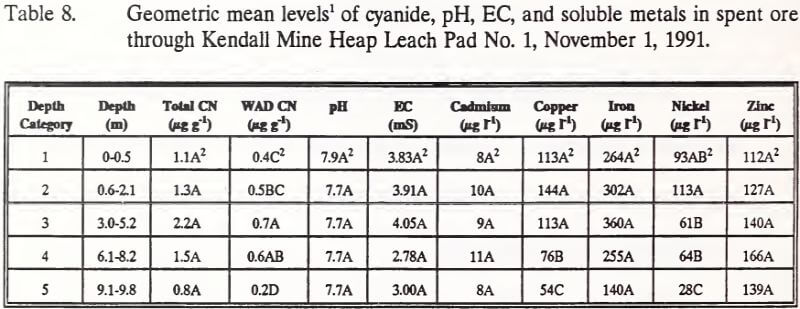
Depth category comparisons of cyanide, pH, EC, and metals means are presented in Table 8. No significant differences exist for total cyanide, pH, EC, cadmium, iron or zinc. WAD cyanide levels increase with depth to a peak in depth categories three and four, followed by a significantly lower concentration in depth increment 5.
Soluble copper concentrations were significantly higher in the upper three depth categories of the heap, subsequently declining through depth categories 4 and 5. Soluble nickel concentrations were similar to copper levels in that
higher concentrations were located near the surface of the heap and decreased with depth.
The peak of WAD cyanide concentrations in depth category three and four (3.0 to 8.2 m) may be attributable to textural changes and/or compaction at a lift interface. These depth categories, approximately halfway through the heap, include the interface between the two lifts comprising Heap Leach Pad No. 1. Schafer et al. (1991) detailed the concept behind particle segregation in heap lift construction that can lead to textural changes at lift interfaces. Coarser ore end-dumped on the second lift rolls to the base where it accumulates over finer ore particles at the top of the first lift (Figure 5). The gradation of each lift, therefore, changes from coarse at the bottom of the lift to finer at the top. The particle size of ore in the upper several feet of each lift may also be reduced by mechanical weathering and particle disaggregation during leaching. Porosity and
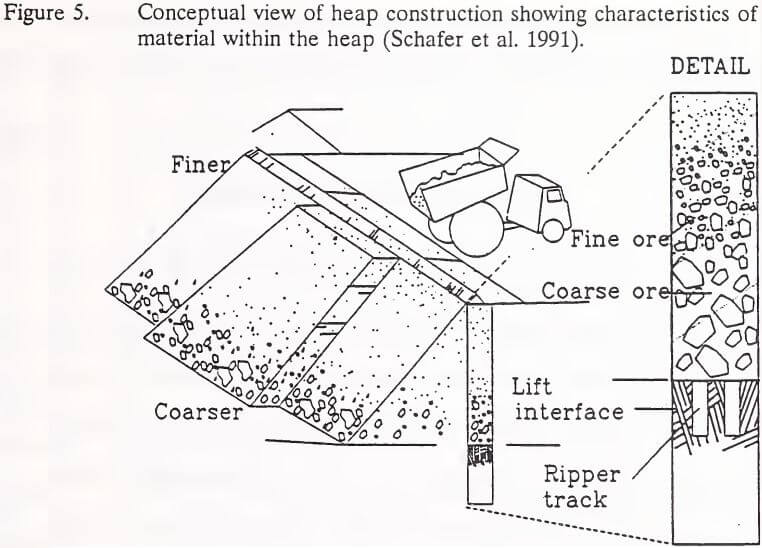
permeability could be further limited in this zone by compaction caused by lift construction traffic (Chamberlin 1981). Changes in flow paths at this interface or the development of blind-offs under the compacted ore may protect residual cyanide from neutralizing rinses and natural degradation processes.
Evidence of the existence of the coarse/fine interface might be found in an examination of the lithological descriptions from split spoon samples from each depth category. Sampling in the interface zone should find rock fragments immediately above fine material. Sampling spoon lithological descriptions from Heap Leach Pad No. 1 do not contain any notation of this distinction. They do indicate rock fragments in 97% of depth categories sampled. However, the descriptions do not differentiate undisturbed whole rock fragments from rock fragments cored from larger rocks and stones by the force of the pounded split spoon.
Cyanide and Metals Areal Distributions
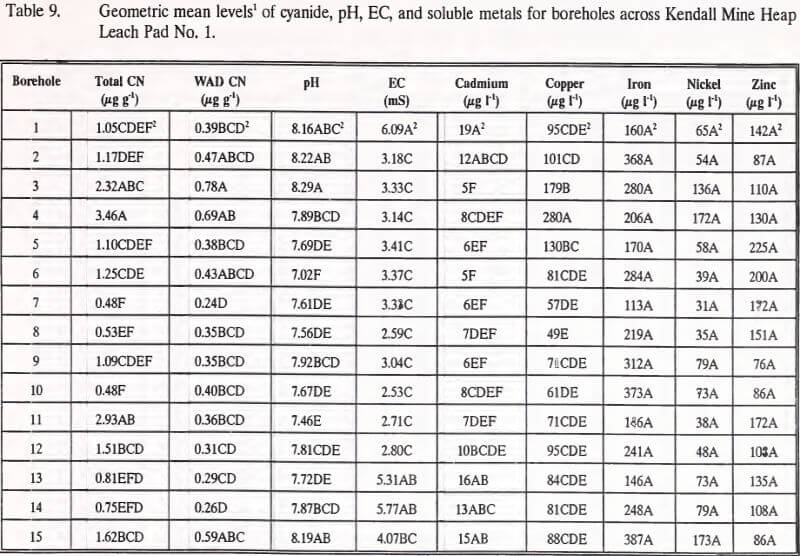
The comparisons of mean cyanide, pH, EC, and soluble metals concentrations for each borehole across Heap Leach Pad No. 1 are summarized in Table 9. Significant differences exist between boreholes for all parameters except soluble iron, nickel, and zinc. However, with one exception, no discernible areal pattern (e.g. mean concentrations changing north to south or east to west across the heap) is apparent among the measured parameters. Only mean borehole cadmium levels exhibited an areal pattern, declining significantly from west to east across the heap.
The distribution patterns, where they exist, for each of the measured parameters is predominantly random. This would indicate that areal patterns have not developed in the neutralized heap. An explanation or hypothesis for these random patterns and the areal pattern of cadmium levels is not possible without control data from the heap prior to leaching and neutralization.
Pore Water Behavior
Pore water samples were collected every two weeks, March through September, 1992. Data from this sampling effort are summarized in Table 10. Pore water WAD cyanide concentrations exceeded Montana’s effluent water quality standard level of 0.22 mg l-1 once over the course of sampling. This measurement, from borehole 4 on August 9, 1992, showed a WAD cyanide concentration of 0.31 mg l-1. The only measurable free cyanide (0.2 mg l-1) was also registered in this borehole on this date. The sample collected from borehole 8 on this date was of insufficient volume for WAD or free cyanide analysis. The previous borehole 4 pore water WAD cyanide concentration was 0.19 mg l-1 on July 24, 1992. Between July 24 and August 9, borehole 4 pore water pH decreased from 7.8 to 6.4, the lowest pH recorded in the sampling program.
Linear regression analysis was used to describe concentrations of parameters in the pore water as functions of time. Results of these analyses and regression coefficients are presented in Appendix C, Tables 43 to 58. Table 11 displays the slope of each regression and associated F and p values. If the slope was different (p < 0.05) from zero, then changes in the concentration of that parameter over the sampling period were demonstrated.
Pore water samples collected from the upgradient position of the heap (borehole 4) showed increased concentrations of total and WAD cyanide, but decreased levels of nickel. No changes were found for these parameters in pore water collected from the downgradient position of the heap (borehole 8).
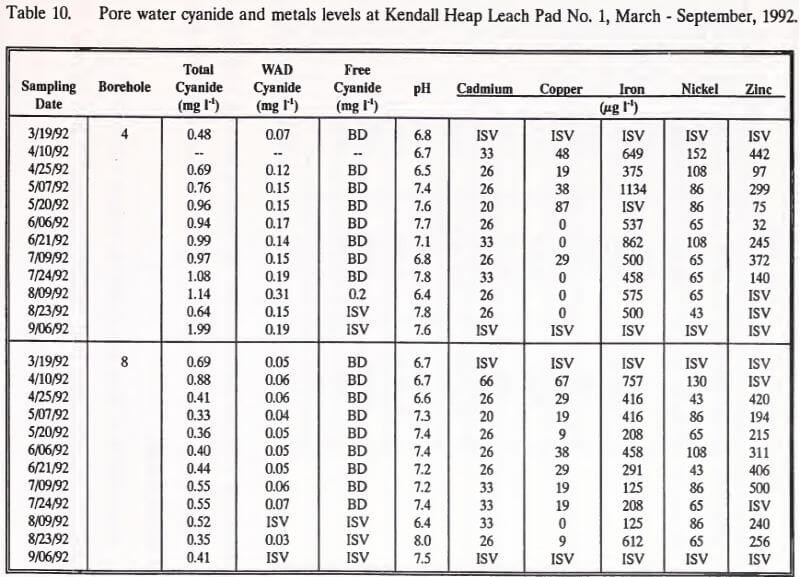
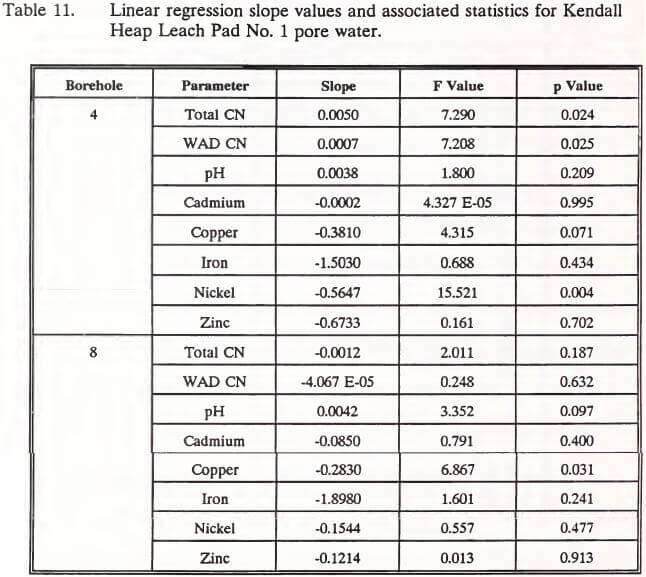
Concentrations of copper decreased over the sampling period in pore waters at the downgradient position. Pore water pH from both gradient positions in the heap did not change.
Interpretation of these data is difficult because they are restricted to one sampling season, and no attempts were made to describe the variation within a particular sampling data or at other positions within the heap. Variation could be measured if multiple pore water samplers were installed in various locations and depths within the heap. The limited data do, however, indicate that upgradient cyanide concentrations increased over the sampling period while downgradient cyanide levels did not change. Cyanide may have become attenuated as pore water drained downgradient over the course of the spring and summer. It is interesting to note that the slopes of the regressions for all metals were negative (Table 11). Factors influencing these decreasing trends may be related to complex stabilities and competing degradation processes described in the literature review.
Summary and Conclusions
In Montana, the use of heap leach cyanidation for gold recovery has become widespread. These heaps, after gold extraction, must be neutralized to state effluent standards. However, cyanide may still exist within a heap, protected from the full effect of neutralization or natural degradation processes. Subsequent drainage from these heaps, particularly in association with large precipitation events, represent a possible threat to downstream water quality.
Cyanide and metal concentrations in ore and pore water from a decommissioned leach heap (Kendall Mine Heap Leach Pad No. 1) were measured and compared. Thirty months after active neutralization with calcium hypochlorite, WAD cyanide levels increased significantly from the heap surface to the middle of the heap, and significantly declined toward the heap bottom. Copper and nickel exhibited a similar vertical distribution. These concentration changes may be attributable to altered flowpaths or blind-off development at a lift interface within the heap. Significant areal distribution patterns could not be derived for cyanide, pH, EC, or metals, with the exception of cadmium, which decreased west to east across the heap. In general, it may be inferred from the data that areal patterns did not develop in the neutralized heap environment.
Examination of the pore water data indicate that total and WAD cyanide concentrations near the bottom of the heap may have degraded as pore water drained downgradient over the course of spring and summer sampling. Pore water copper and nickel concentrations declined over the sampling period. These changes might be due to relative metallo-cyanide complex stabilities and competing degradation processes.
Conclusions regarding the distribution of cyanide within this heap cannot be made without recognition of the difficulty in measuring cyanide concentrations in heterogeneous spent ore. Development and standardization of collection protocols and analysis procedures for cyanide in spent heap ore, pore water, and effluent would be helpful to both industry and regulators in the evaluation of neutralization efficacy. Nevertheless, within the limitations imposed by the analytical techniques available for the determination of cyanide concentrations in solids, it may be inferred from the data that areal patterns did not develop in the neutralized heap.
