Table of Contents
In accordance with a program for developing new materials required for emerging technologies, an investigation was undertaken to determine the properties of dispersion-strengthened copper. The copper contained either alumina or yttria as a dispersoid and was prepared by coprecipitation-a potential method for commercial production. Although the coprecipitation method had been used previously to prepare dispersion-strengthened copper, neither had the properties of this material been fully evaluated, nor had yttria been investigated as a dispersoid. Also, the effects of varying compositions and fabrication factors had not been fully evaluated.
Earlier studies had shown that copper strengthened by a dispersion of very fine inert oxide particles possesses a unique combination of properties; it resists softening and grain growth after being heated to temperatures near the melting point of copper, it is more creep resistant at elevated temperatures than oxygen-free copper, and it has electrical and thermal conductivities nearly as high as oxygen-free copper. The conservation of copper would be aided by substituting thinner sections of dispersion-strengthened material in applications where heavy sections of copper are now used.
One immediate application for dispersion-strengthened copper could be in microwave tubes, whose manufacture utilizes a considerable amount of oxygen- free copper and requires brazing at high temperatures. These temperatures cause recrystallization, grain growth, and strength loss in oxygen-free copper. Since microwave tubes must maintain close dimensional stability while enclosing a high vacuum, heavier sections of copper must be used in their design than would be necessary if a stronger material were used. New types of tubes and similar devices could also be designed and built if a suitable material were available.
Other possible applications include large motors and generators, switches, current-carrying springs, transmission lines, spot welding electrodes, magnet windings, die casting mold core pins, heat exchangers, and friction plates for disk brakes.
The preparation of dispersion-strengthened copper has been given attention by several investigators. Zwilsky and Grant mixed copper and alumina powders of several particle sizes to produce alloys with alumina content ranging from 2.5 to 10 vol pct; Preston and Grant made copper-alumina alloys (up to 3.5 vol pct oxide) and copper-silica alloys (up to 12 vol pct oxide) by compacting and extruding internally oxidized, powdered, dilute solid solutions of silicon and aluminum in copper. The best test properties were obtained with copper-alumina alloys.
Jackson prepared a copper-alumina alloy by coprecipitation from aqueous nitrate solutions; the same method was also described by Grimwade and Jackson. Iler, Pasfield, and Yates have reported a coprecipitation method using colloidal metal oxide aquasols as a source of dispersoid to produce copper dispersion strengthened with zirconia, silica, alumina, and thoria.
A copper-beryllia alloy, formed by internal oxidation, has been described by McDonald. Das, Chevrette, and Freedman, also using the internal oxidation method, prepared a copper-alumina alloy for electron tube applications.
A melting and casting method for dispersion-strengthened copper would be most desirable from the standpoint of convenience and economy of production. However, published work has indicated that this method has not been developed to the point where the properties or microstructure of the material produced are comparable to those obtained by any of the powder metallurgical processes. London described the formation, by this liquid metal process, of a 2 and 4 percent thorium boride dispersion.
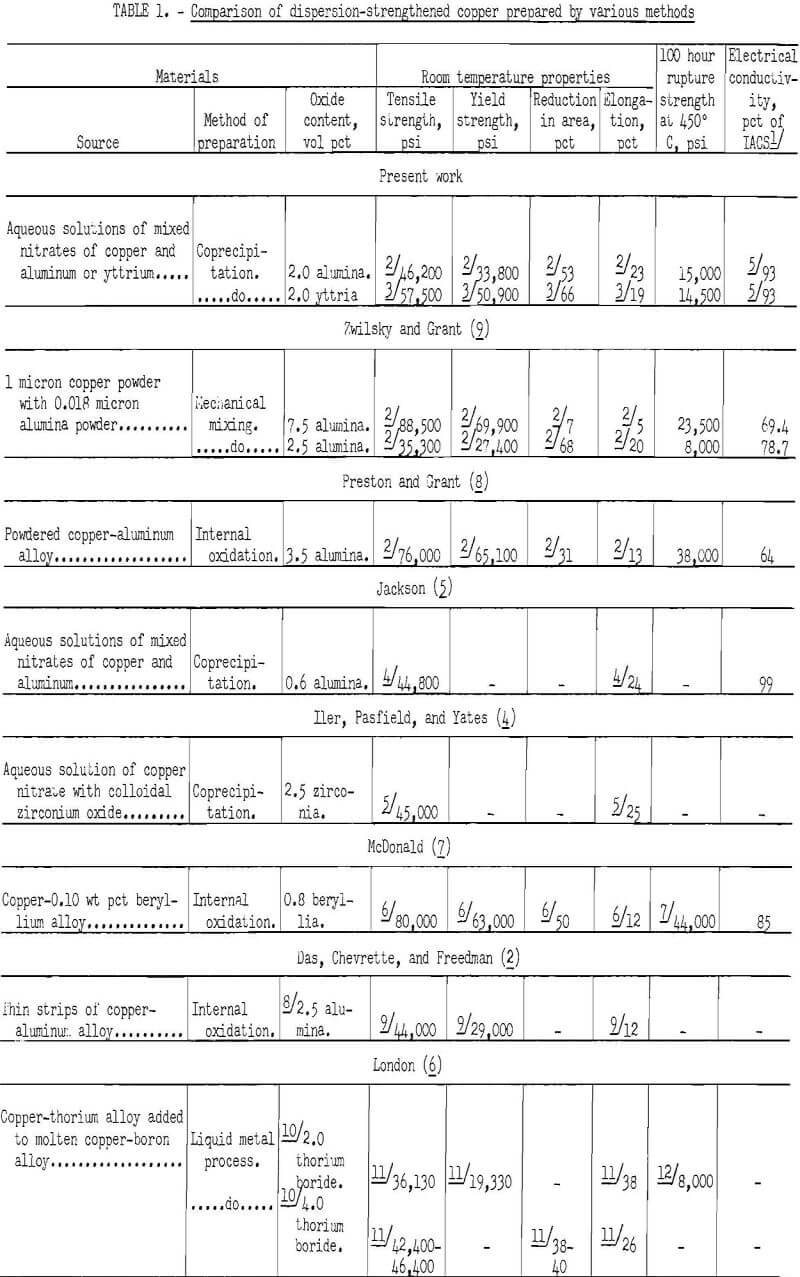
The Bureau of Mines prepared dispersion-strengthened copper by coprecipitation of aqueous solutions of mixed nitrates of copper and aluminum or yttrium with ammonia, conversion to oxides, hydrogen reduction, compaction, and extrusion. Some of the extruded rod was cold swaged. A comparison of the properties of dispersion-strengthened copper from the present investigation and from various sources as reported in the literature is shown in table 1. Tensile properties shown in the table are only roughly comparable, since they were obtained for materials given different mechanical and thermal treatments in the various investigations. Rupture strength at 100 hours and 450° or 400° C is also shown for those studies in which it was reported. The tensile and stress-rupture strengths of the materials in the present work are not as high as the maximum values that are shown for some of the other studies. However, no attempt was made to maximize the properties in the present work.
The coprecipitation method of preparing dispersion-strengthened copper has numerous advantages which can best be shown by listing the disadvantages of other methods. The mechanical mixing method, for example, has the following disadvantages:
- It is difficult to produce a good dispersion without agglomeration of the oxide particles, which tends to cause segregation and lamination in the working direction of the fabricated material.
- Mixing of powders on a large scale may prove to be difficult.
- Dispersoid particle spacings are limited by the copper particle size
- The size of the dispersoid is limited by the powders that are available
- The relative sizes of the copper and oxide particles must be carefully regulated for best mechanical properties.
- The dispersoid particles are generally present only at the grain boundaries of the copper, a condition which reduces ductility.
The internal oxidation method has the following disadvantages:
- This process is limited by the diffusion rate of oxygen in solid copper. Thin sheet, wire, or powder must generally be used.
- Control of the particle size is difficult. The initial precipitate particles tend to grow as the process proceeds
- There is a tendency for the diffusing oxygen to form copper oxides in the grain boundaries. This means that the material is subject to hydrogen embrittlement if brazed in a hydrogen atmosphere.
The coprecipitation method has the following advantages:
- The process could be adapted to commercial operations involving leaching of primary or secondary copper sources.
- The process produces well-dispersed particles in the optimum size range for strengthening.
- Concentration of oxide can easily be varied.
- The process is adaptable to a variety of oxides.
- The plastic deformation that is required to produce the optimum properties can be introduced during the compaction and extrusion of the powder, and by subsequent cold work.
Preparation
Coprecipitation
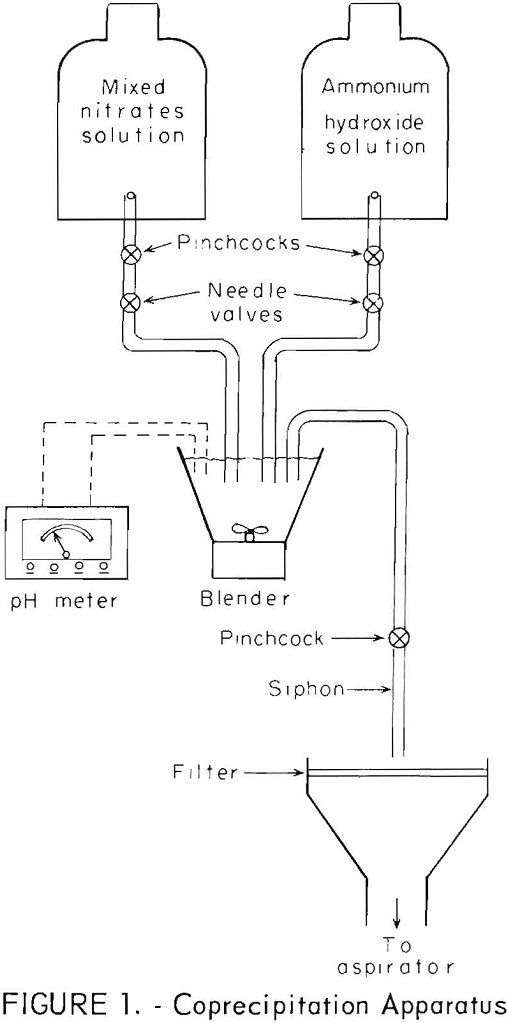
Analyzed water solutions of cupric nitrate and aluminum nitrate were combined to give a final solution with a concentration of 145 g/l of copper and 0.61 g/l of aluminum. This solution was coprecipitated with a 5 molar solution of ammonium hydroxide in the apparatus shown in figure 1. The solutions were run simultaneously into a high-speed blender, which was initially filled with distilled water. The pH was monitored throughout the runs by means of a pH meter and maintained between 5.5 and 6.0 by adjustment of the needle valves. The precipitate was transferred to a filter by means of a siphon. About 7 liters of mixed nitrate solution per run was used to give a precipitate with a copper content of about 1 kilogram. The precipitate was washed twice with distilled water, filtered after each wash, and then aspirator dried at 100° C for 24 hours or more. The composition of the precipitate was identified by X-ray diffraction analysis as Cu2(OH)3NO3- the aluminum compound was present in insufficient quantity to be identified. The same procedure was followed with a solution of mixed cupric and yttrium nitrates, except that the solution contained 145 g/l of copper and 1.26 g/l of yttrium; the pH was maintained at 6.8. The ratios of copper to aluminum and copper to yttrium were chosen to produce a final composition of the dispersion-strengthened copper of 2 vol pct of alumina or yttria respectively. Chemical analysis of the final material gave a mean value of 2.07 vol pct with a standard deviation of 0.09 for the copper-alumina, and a mean value of 2.04 vol pct with a standard deviation of 0.03 for the copper-yttria.
Air Firing and Hydrogen Reduction
The dried precipitates were sequentially heated in air at 200° C for 1 hour, 500° C for 1 hour, and 800° C for 1 hour, to drive off combined water, oxides of nitrogen, and residual salts, and to convert the precipitate to a mixture of cupric oxide and either aluminum oxide or yttrium oxide. Gradual heating was used to prevent sudden expulsion of vapors and loss of powder. After air firing, the copper oxide was reduced in a hydrogen furnace. Since the reduction is exothermic and must begin at low temperatures to prevent sintering of the copper powder, the temperature of the hydrogen furnace was slowly raised to 200° C, held for 1 hour, followed by heating to 250° C and holding for 1 hour and then heating to 800° C and holding for 10 minutes. The reduction reaction was substantially complete at 250° C but the higher temperatures were used to remove the last traces of oxygen, which could cause blistering or cracking at later stages of fabrication. After reduction, the powder was processed in a high-speed blender to eliminate caking.
Pressing and Sintering
The 1-kilogram batches of powder were pressed in a double-acting die at pressures between 20,000 and 51,000 psi, except for two batches of copper-alumina which were each divided into 2 parts and isostatically pressed at 8,000 to 8,500 psi. The pressed billets were 2 inches in diameter.
The billets were sintered in hydrogen at 900° C, allowing 2 hours to reach temperature and 1 hour at temperature. For billets pressed in the double-acting die, the pressed density ranged from 68 to 74 percent of theoretical; these density values increased by 6 percent or less after sintering. The isostatically pressed billets had densities of 43 to 55 percent of theoretical after pressing and these values increased by 5 to 14 percent after sintering.
Extrusion and Swaging
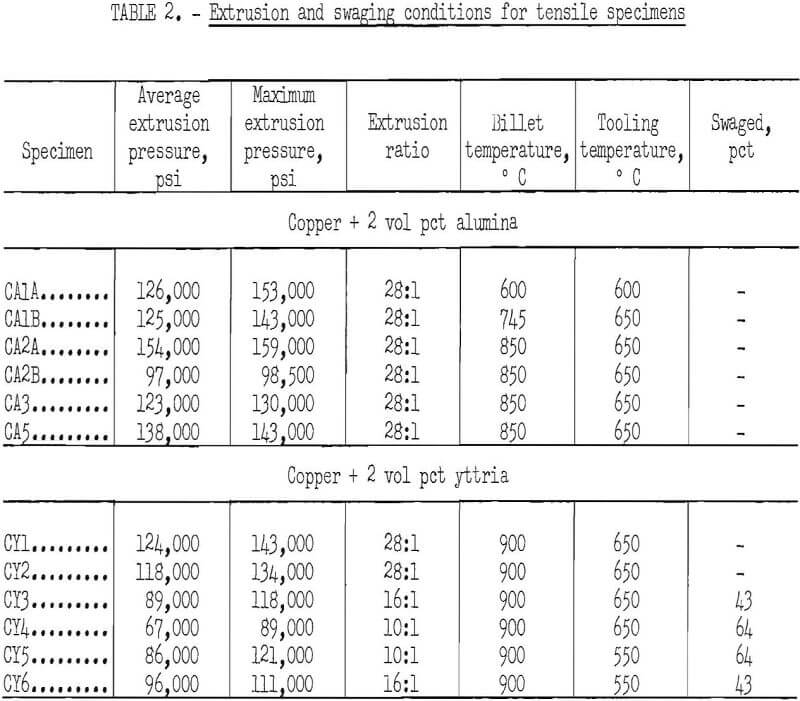
Rods were extruded in a 500-ton vertical hydraulic press at extrusion rates of about 2.5 feet per minute. Higher extrusion pressures were found to be necessary than for extruding copper without dispersoid. The tooling (container, die, and follower block) was preheated in an air furnace, while the copper billet was preheated in a hydrogen-atmosphere furnace. After a period of trial and error, satisfactory preheating temperatures of 650° C for the tooling and 900° C for the billet were established. It was found desirable to coat the exterior of the container with an antiscaling compound and the interior with molybdenum disulfide to reduce friction. Because of rapid heat losses, the actual temperature of extrusion was not known. Although extrusion was started as rapidly as possible after the billet and tooling were removed from the preheating furnaces, small differences in timing could have influenced the actual extrusion temperature.
Extrusion pressures were measured every 15 seconds and the average value determined. The maximum pressure (usually attained at the end of an extrusion) was also recorded. The 2-inch-diameter billets were extruded to 3/8-inch-diameter rod (extrusion ratio 28:1) for testing without swaging. Rods that were to be cold swaged were extruded from a 2-inch-diameter billet to either ½ inch (extrusion ratio 16:1) or to 5/8 inch (extrusion ratio 10:1). The ½-inch and 5/8-inch rods were cold swaged to 3/8-inch by 0.025-inch steps without intermediate annealing, resulting in reductions in area of 43 and 64 percent, respectively.
Properties
Tensile and Hardness Measurements
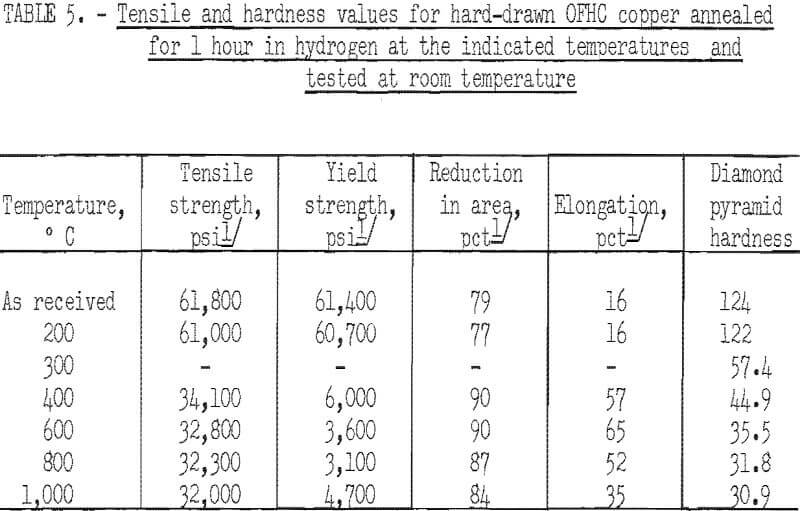
Tensile specimens for testing at room temperature were 3/16 inch in diameter with a ¾-inch gage length. Tensile specimens were made from several different batches of the same composition that were extruded under varying conditions. In each batch, where sufficient specimens were available, a specimen was annealed for 1 hour in hydrogen at each of the following temperatures. 200°, 400°, 600°, 800°, and 1,000° C. Where insufficient specimens were available, one specimen was annealed at 600° C and one at 1,000° C. Tensile tests were made on the specimens in both the as-extruded or as-swaged condition and in the annealed condition. Specimens from commercial hard-drawn 3/8-inch OFHC² copper rod were similarly annealed and tested. Tensile strength, yield strength at 0.2 percent offset, reduction in area, and elongation were measured. Vickers hardness (DPH-10 kg load) measurements were made on the shoulders of the broken tensile specimens, or on pieces of extruded rod in cases where tensile specimens were not tested.
The results are shown in tables 3 to 5, and selected values of hardness, tensile strength, and yield strength are plotted in figures 2 to 9. (Extrusion conditions of the test specimens are shown in table 2.) The tensile values represent tests from a single specimen at each temperature, except for the OFHC copper for which the mean of three specimens is recorded. The hardness values are the means of five impressions from each specimen.
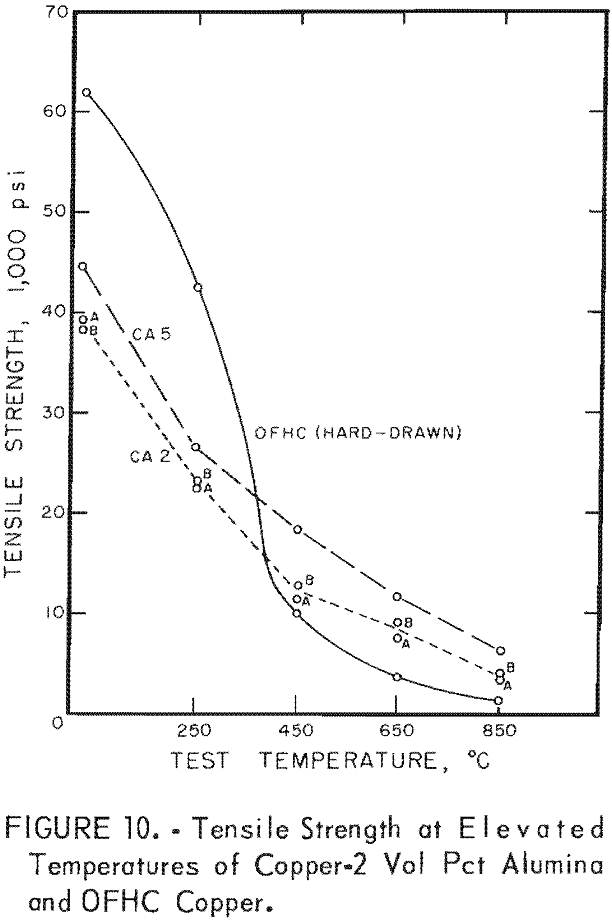
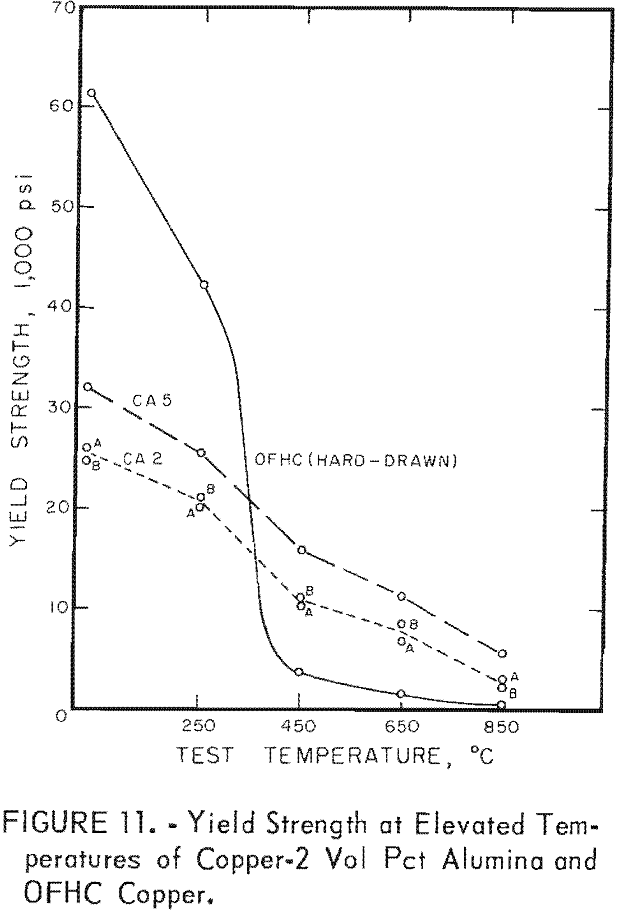
For hot tensile tests, 3/16-inch-diameter specimens with a 1.05-inch gage length were used. Tensile tests at 250°, 450°, 650°, and 850° C were made on two extrusions from one batch and one extrusion from another batch of copper 2 vol pct alumina specimens in the as-extruded condition, and on commercial hard-drawn OFHC copper. The data are shown in table 6 and in figures 10 and 11. The tensile values represent a single test at each temperature for batches CA2A and CA2B, the mean of two tests at each temperature for batch CA5, and the mean of three tests at each temperature for the OFHC copper. Tests were conducted in air at 250° and 450° C and in helium at 650° and 850° C.
Stress-Rupture Measurements
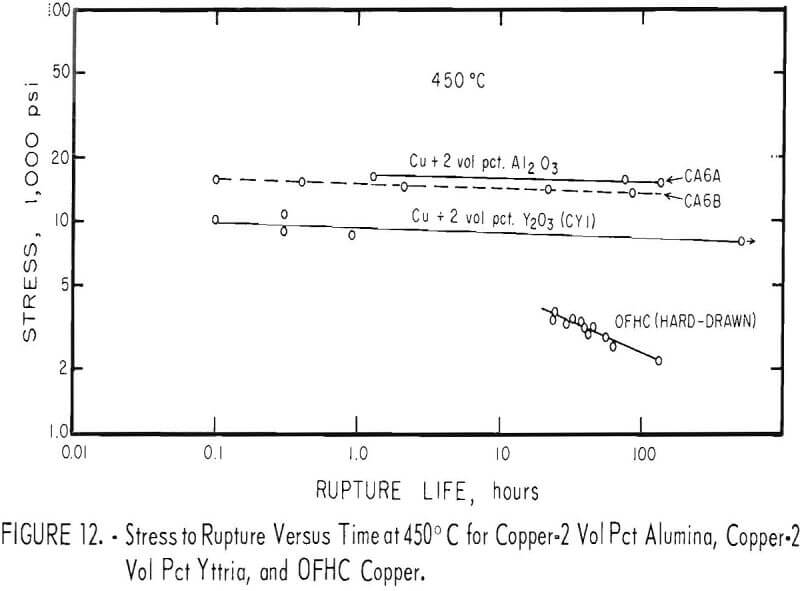
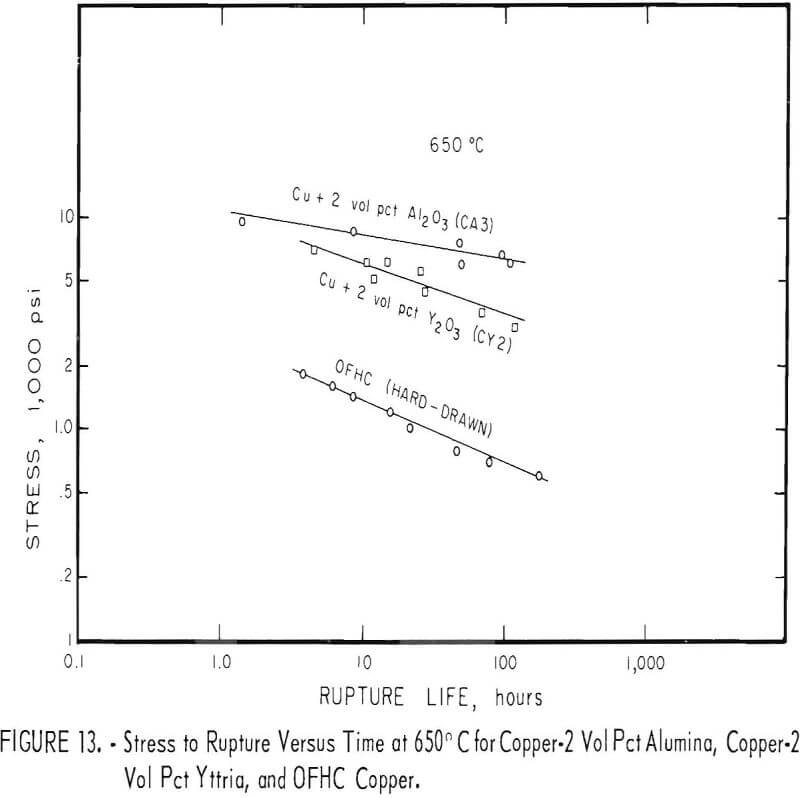
Stress-rupture test specimens were 3/16 inch in diameter with a ¾-inch gage length. Stress-rupture tests at 450° C were run on one batch of copper-2 vol pct alumina (pressed and extruded in two sections) one batch of as-extruded copper-2 vol pct yttria, and a series of four extruded and swaged copper-2 vol pct yttria alloys. Commercial OFHC copper, in the form of 3/8-inch hard-drawn rod, was also tested for comparison. Tests were also run at 650° C on one batch of copper-2 vol pct alumina, one batch of copper-2 vol pct yttria, and on OFHC copper. At 850° C, tests were run only on one batch of copper-2 vol pct alumina (pressed and extruded in two sections). Tests were run in air at 450° C and in flowing nitrogen at 650° and 850° C. The results were plotted (figs. 12 and 13) as the logarithm of stress versus the logarithm of time at the testing temperature. Values of 100-hour rupture stress were read from graphs similar to figures 12 and 13 for all the alloys tested, and are shown with the extrusion conditions in table 7.
Electrical Conductivity
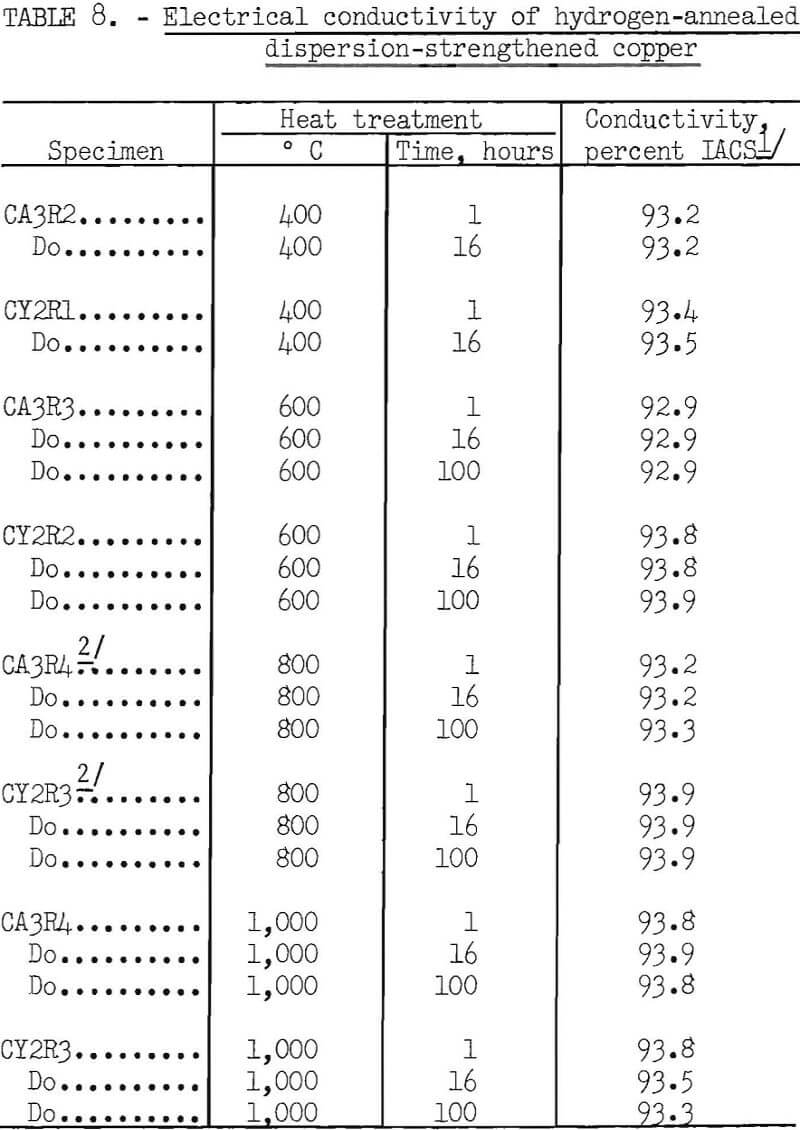
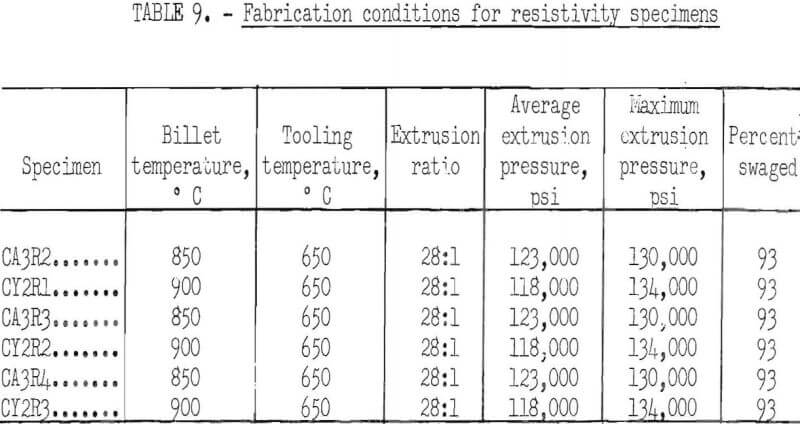
Specimens for resistivity measurements, which were 0.1 inch in diameter by 8.5 inches long, were each annealed in hydrogen for 1 and 16 hours at 400° C, and for 1, 16, and 100 hours at 600°, 800°, and 1,000° C. The specimens heat treated at 1,000° C had previously been annealed at 800° C for 100 hours. Resistivity measurements were made over a gage length of 8.118 cm with a Kelvin double bridge, and the specimens were maintained at 20°±0.1° C in an
oil bath during measurement. The resistivity values were converted to conductivity in percent LACS. The precision of the individual resistance measurements, as expressed by 95 percent statistical confidence limits, was ±0.16 microhm, which is equivalent to ±0.06 percent LACS for the specimens measured. The results are shown in table 8 and the fabrication conditions for the specimens in table 9
Density
The density of copper-2 vol pct alumina was calculated from accurately determined weights and dimensions of extruded and machined cylinders that were ¼ or 3/8 inch in diameter and 4 inches long, from three batches of the material. The density of the three specimens was found to be identical at 8.74 g/cm³, which is 98.6 percent of the theoretical value of 8.86 g/cm³ for material of this composition. The density of OFHC copper was similarly measured and found to be 99.7 percent of theoretical.
Discussion
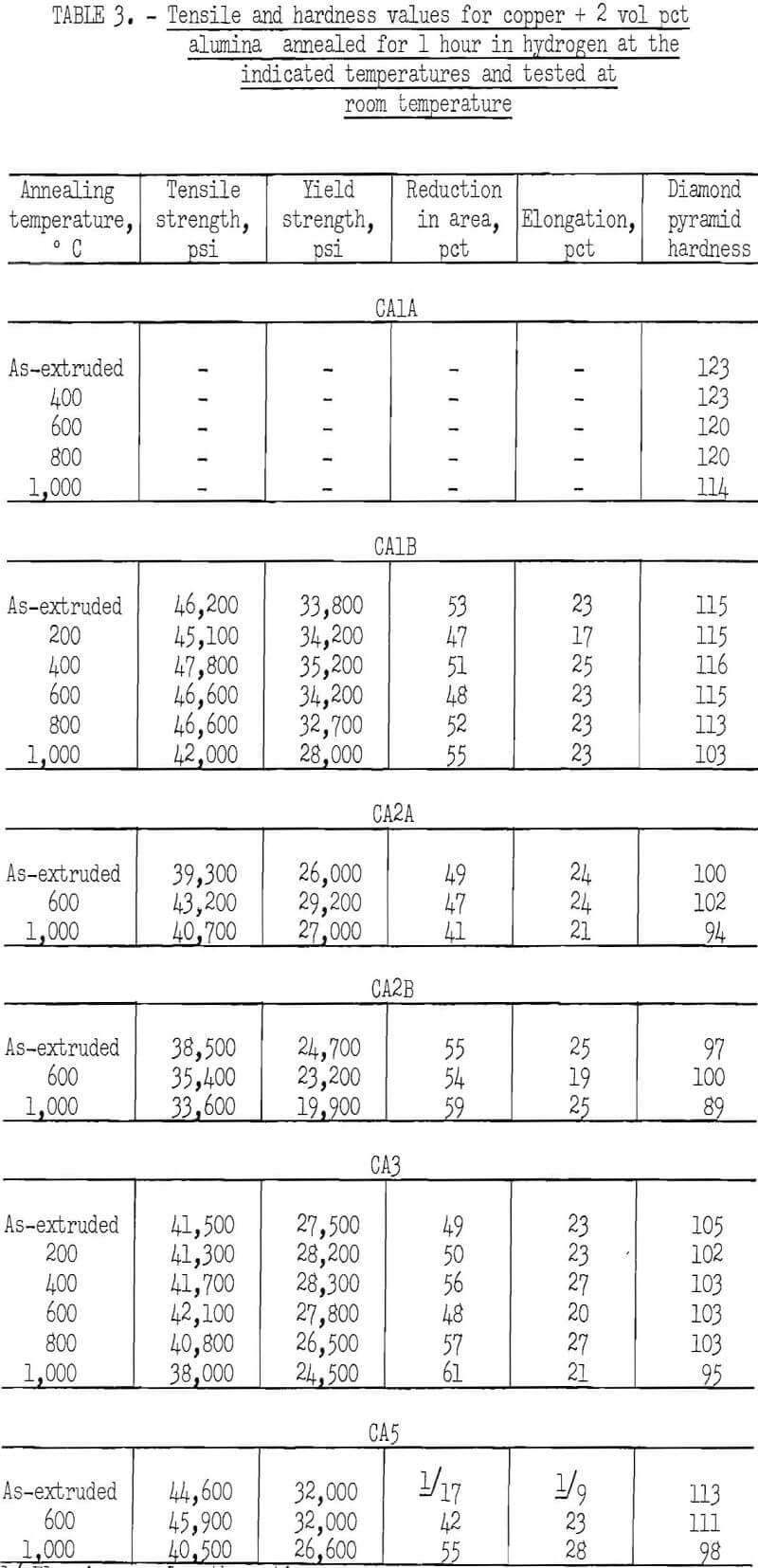
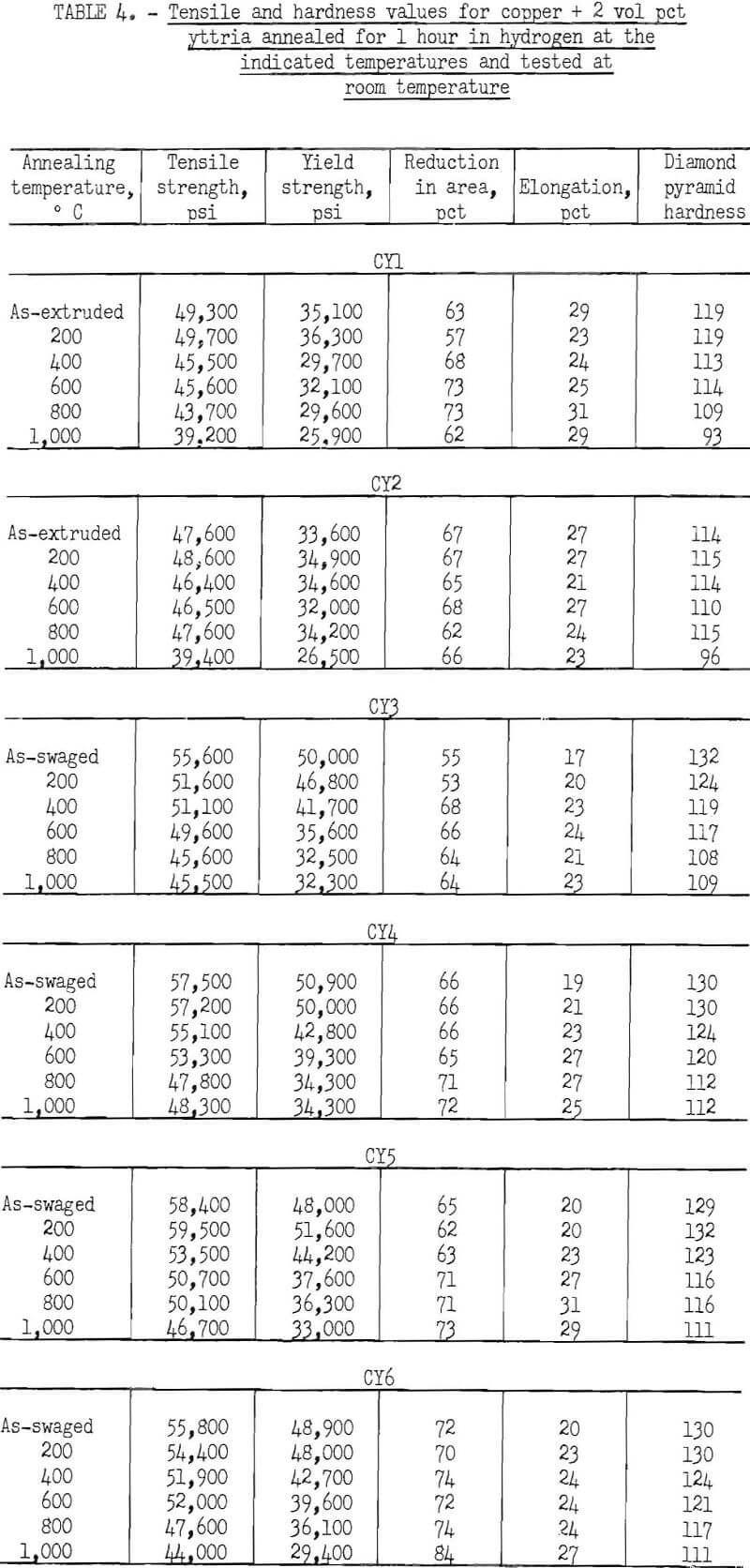
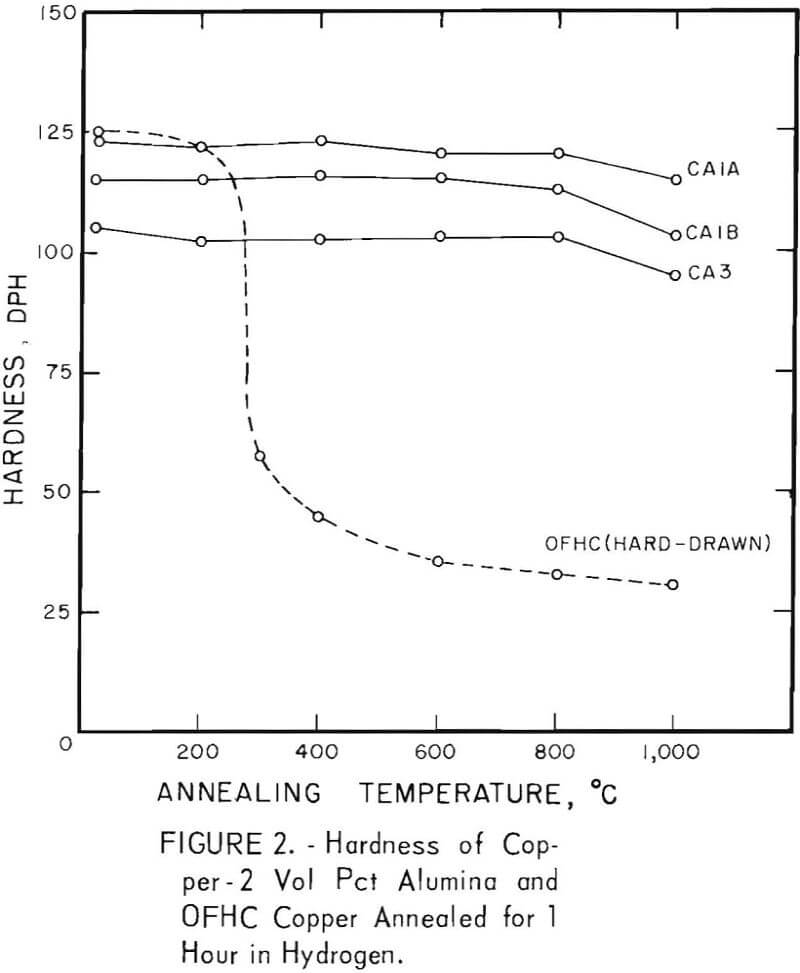
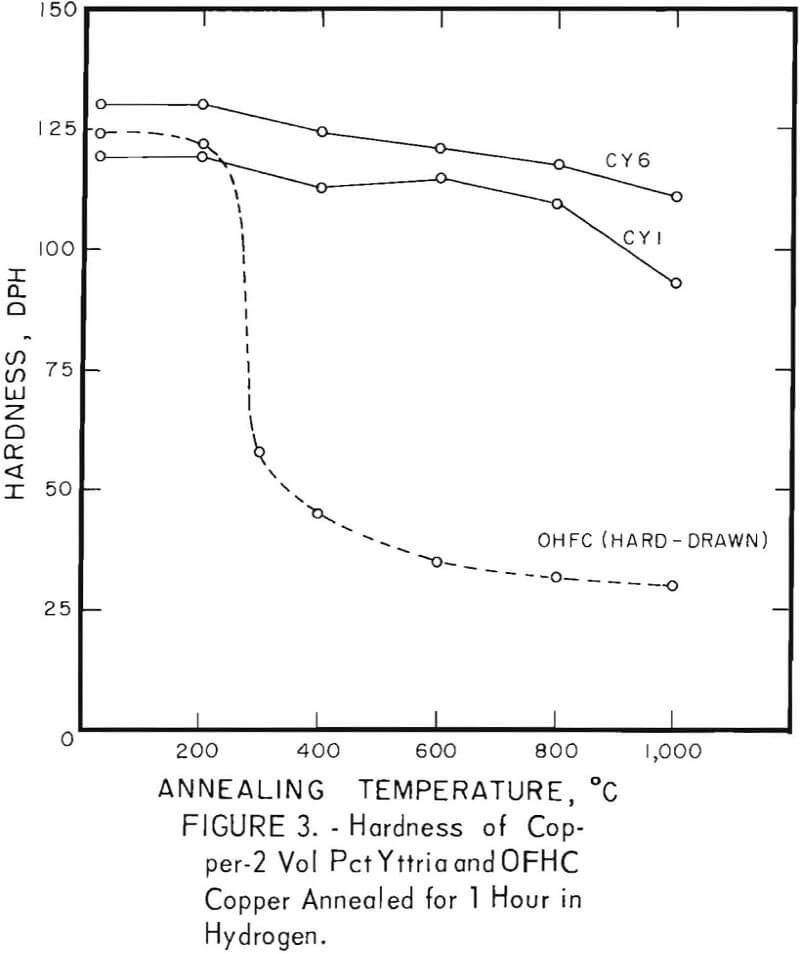
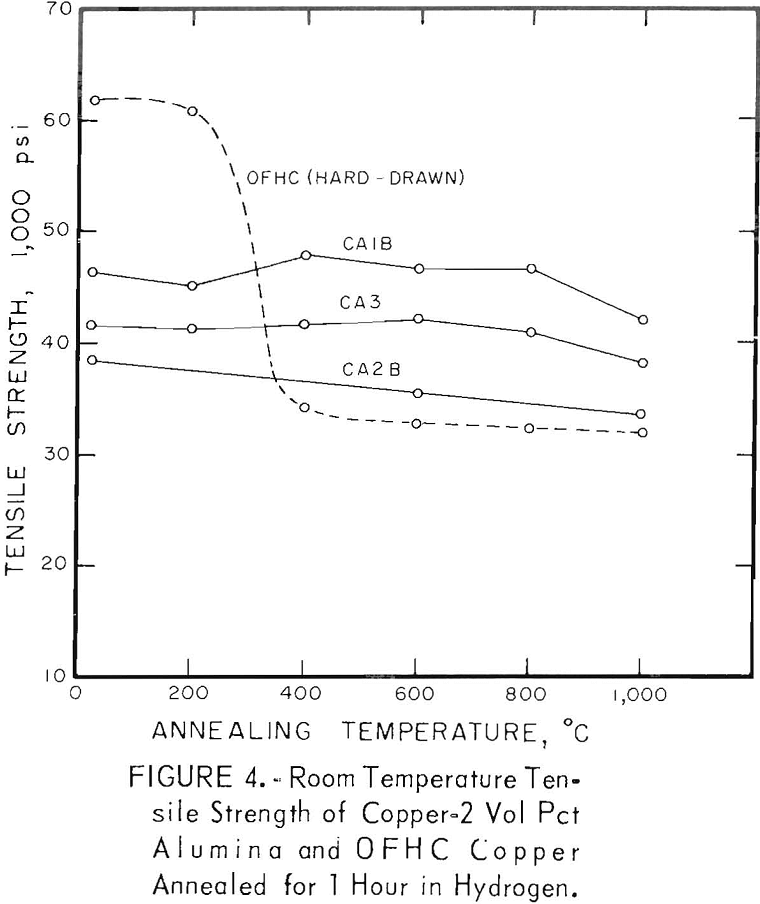
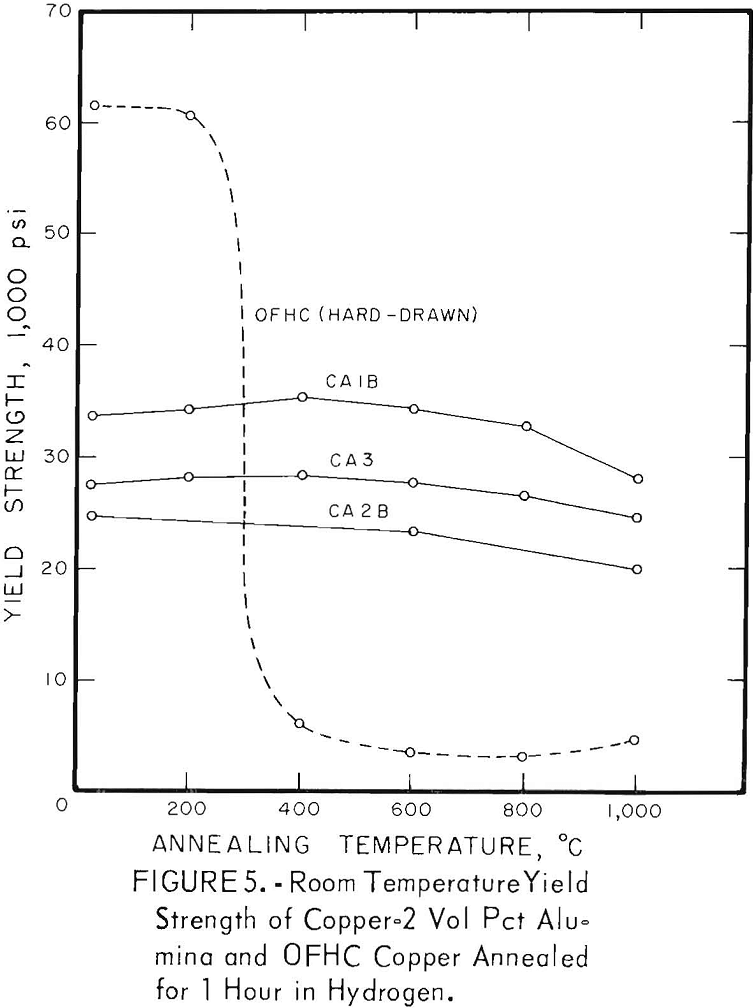
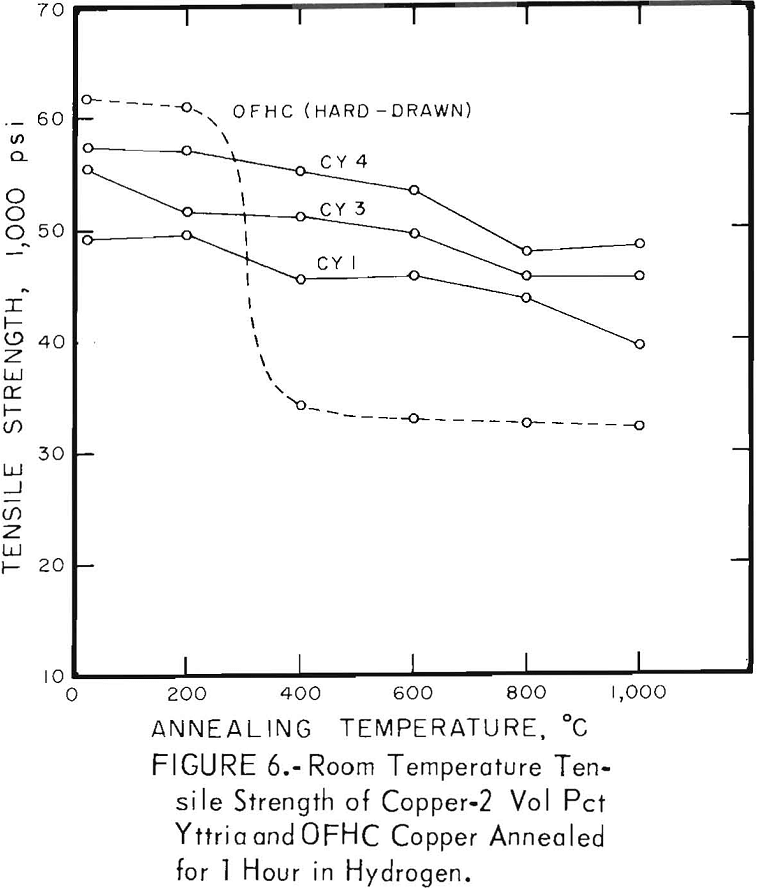
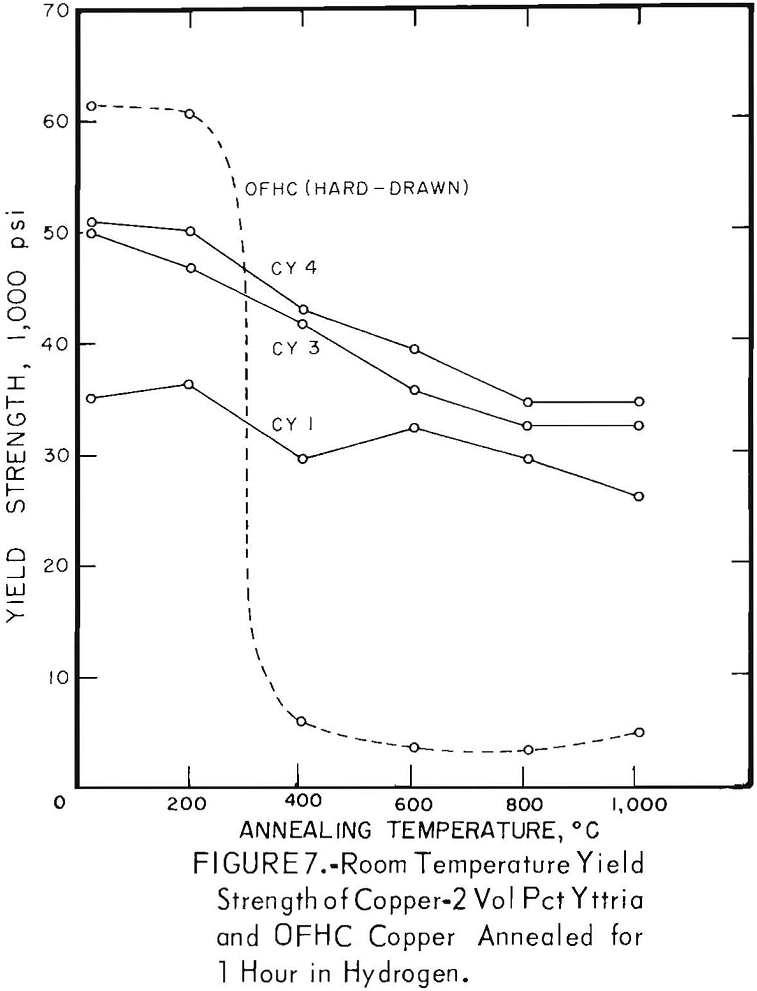
The characteristic property of dispersion-strengthened copper in resisting annealing is shown by the curves in figures 2 to 9 and the data in tables 3 and 4. These data show that, compared to hard-drawn OFHC copper, both the copper alumina and the copper yttria alloys decreased much less in strength and hardness with increasing annealing temperature and were harder and stronger after annealing at all temperatures above 400° C (figs. 2 to 7).
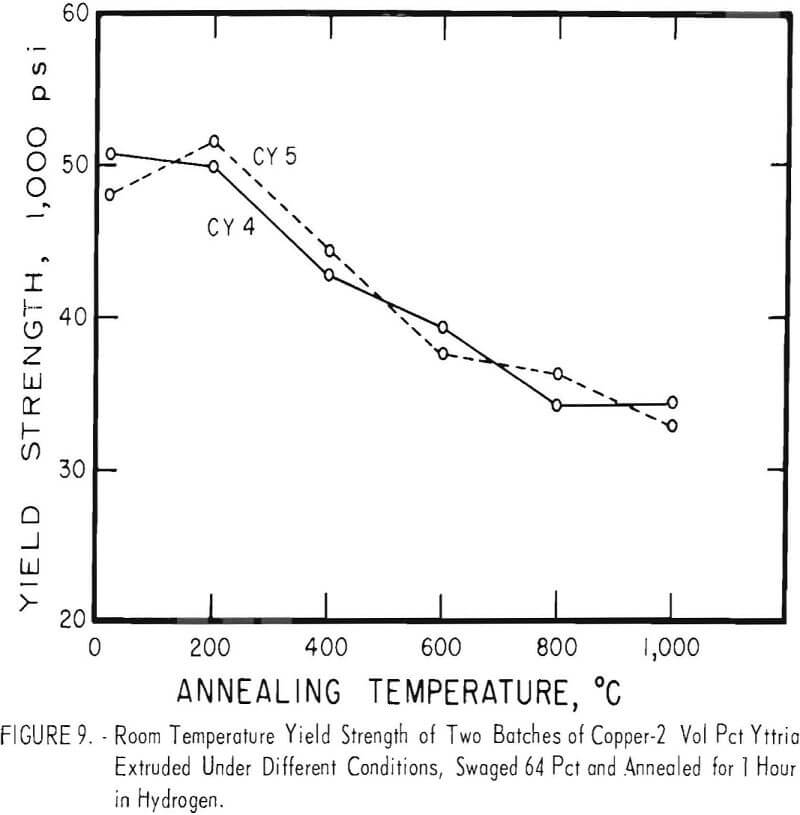
Even after heating for 1 hour at 1,000° C, the dispersion-strengthened copper still retained a large proportion of its original room temperature strength and hardness. Hard-drawn OFHC copper begins to soften at annealing temperatures above 200° C and has lost essentially all of the strength of work-hardening by 400° C. The contrast between dispersion-strengthened copper and OFHC copper is most marked in the yield-strength behavior (figs. 5 and 7).
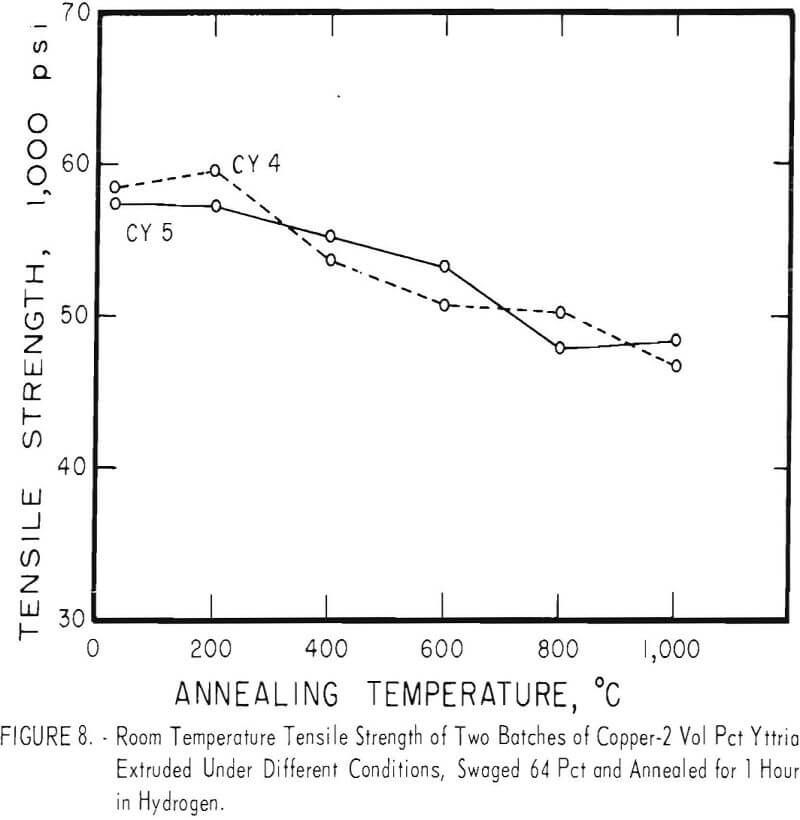
Cold swaging raised the strength and hardness of the copper-yttria alloy (the copper-alumina alloy was not swaged), and a greater reduction by swaging produced a greater strength increase. These differences were apparent both in the as-extruded or as-swaged conditions and in the annealed condition (figs. 3, 6, and 7).
The tensile ductility of the dispersion-strengthened copper measured by reduction in area and elongation is satisfactory in all conditions, although it is somewhat lower than that of annealed OFHC copper. The values of reduction in area were about 50 to 55 percent for the copper-alumina and 60 to 75 percent for copper-yttria, as compared with 79 percent for hard-drawn OFHC and 90 percent for OFHC copper annealed at 400° C. The elongation values were around 20 to 25 percent for both copper-alumina and copper-yttria, as compared with 16 percent for hard-drawn OFHC and 57 percent for OFHC annealed at 400° C.
There was considerable difference in the strength and hardness of different batches having the same nominal composition, but which were extruded separately under varying conditions (figs. 2, 4, and 5). In general, it appears that those alloys that were extruded at the lowest temperature and with the highest extrusion pressure, had the highest strength and hardness. However, the conditions of pressure and temperature of the material as it passed through the extrusion die were unknown, and it is these conditions that undoubtedly affect the properties of the material. The preheat temperatures of the tooling and billet and the measured extrusion force can only be used as a rough guide to these conditions,
A series of four copper-yttria alloys (CY3 to CY6) was swaged after extrusion under different conditions of extrusion temperature and pressure to determine whether the properties could be equalized by swaging the extruded rod by an equivalent amount. Figures 8 and 9 show that the tensile and yield strength of alloys extruded under different conditions and then swaged 64 percent are approximately the same. Similar results were found for the alloys swaged 43 percent. It therefore appears that the cold work of swaging essentially eliminates differences due to small variations in extrusion conditions, and that the properties of similar batches of material that have been swaged an equal amount can be directly compared.
The results of hot tensile tests (figs. 10 and 11 and table 6) show that dispersion-strengthened copper retains a higher strength at testing temperatures of 450° C and above than does OFHC copper. Hot tensile tests were run only on copper-alumina alloys, mainly to determine starting stress levels for stress-rupture tests. Some variations in hot tensile properties as well as room temperature tensile properties are apparent between batches. Stress- rupture tests were considered more meaningful criteria of elevated temperature behavior than short-term hot tensile tests; therefore, only stress-rupture tests were conducted on the copper-yttria alloys.
The copper-alumina and copper-yttria alloys were much superior to OFHC copper in stress-rupture tests at 450° and 650° C. The dispersion-strengthened copper also retained some strength at 850° C, whereas hot tensile tests indicated that the strength of OFHC copper at this temperature is so low that stress-rupture tests would have little meaning. The 100-hour rupture strength of swaged copper-yttria was improved over that of the as-extruded material. However, the as-extruded copper-alumina alloys had about the same rupture strength as the swaged copper-yttria alloys.
Resistivity measurements have shown that dispersion-strengthened copper has a comparatively high value of conductivity relative to the International Annealed Copper Standard. This conductivity is maintained in both copper- alumina and copper-yttria even when the alloys are annealed in hydrogen for as much as 100 hours at temperatures as high as 1,000° C (table 7).
Density measurements on copper-alumina showed values that were somewhat below the theoretical values based on a mixture of copper with the dispersoid. This lowered density probably results from the porosity that was noted in the microstructure of all specimens.
Polished and etched sections made from the broken tensile specimens and examined by light microscopy showed that all specimens had a similar structure, whether they contained alumina or yttria as the dispersoid, and regardless of the annealing temperature. Figure 14 shows typical structures for transverse

sections of copper-2 vol pct alumina and copper-2 vol pct yttria, as-extruded and after annealing at 1,000° C for 1 hour in hydrogen. The structure is very fine grained and does not show any recrystallization or grain growth at annealing temperatures up to and including 1,000° C. All specimens had pores or voids in the microstructure, and the dark spots that appear in figure 14 are typical of the smaller voids. There were also occasional, much larger pores in most of the specimens. The origin of these voids is at present unknown. Two possibilities are that they originate from agglomerations of the dispersoid that are torn out during metallographic preparation, or that they represent entrapped gas that prevented complete densification during processing.
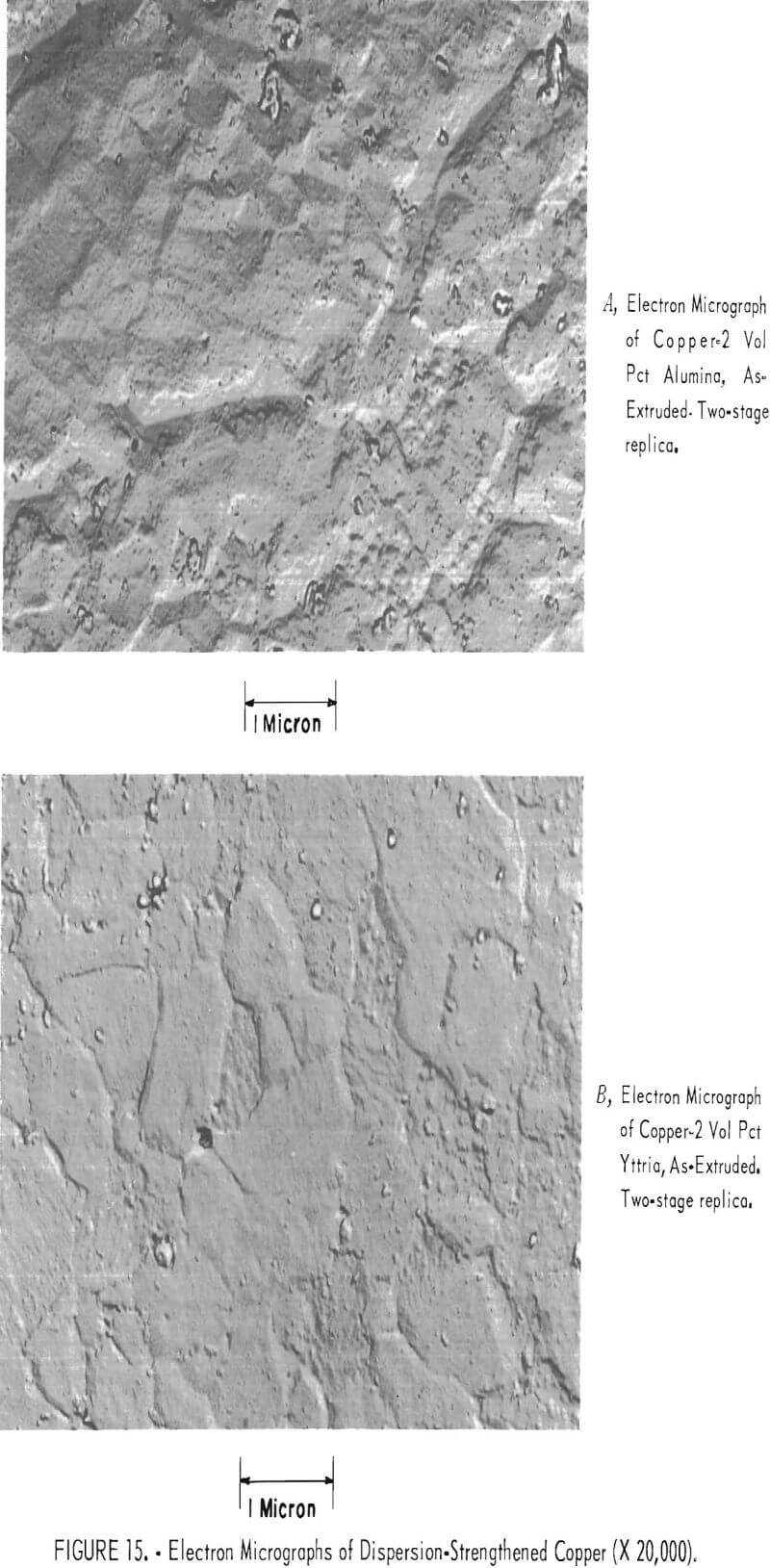
Electron micrographs of typical specimens are shown in figure 15. The very fine particles, which are of the order of 200 to 1,000 angstroms in diameter, are in the correct size range to be effective in dispersion-strengthening. The micrographs also show large particles, which may be agglomerated oxides. Because of the porosity and possible agglomeration of some of the oxides, it is unlikely that maximum strengthening was attained for the amount of dispersoid added.
Conclusions
- Copper, dispersion-strengthened with alumina or yttria by a co-precipitation process, has strength and hardness superior to OFHC copper when these materials are compared as hydrogen-annealed between 400° and 1,000° C
- Above about 400° C, elevated temperature strength and stress-rupture strength of the dispersion-strengthened copper are also higher than those of OFHC copper
- In general, extruding at a higher pressure or lower extrusion temperature produces material with better mechanical properties.
- Cold swaging improves the properties of the extruded dispersion-strengthened copper, and tends to eliminate differences due to variations in extrusion conditions.
- The electrical conductivity of the dispersion-strengthened copper was about 93 percent IACS and was not changed by hydrogen annealing.