Sulfide ores are generally concentrated by grinding with steel media followed by flotation. Interactions between the grinding media and sulfide minerals adversely affect the floatability of the sulfides, increase the corrosive wear of the grinding media, and result in unrecovered sulfides in the flotation tailings leading to a loss of natural resources and the release of heavy metal ions in tailings ponds. In order to overcome these effects, it is necessary to establish the mechanism by which the surface properties of sulfide minerals are altered by interactions with grinding media.
Materials and Methods
The galena used in this investigation was from Brushy Creek, Missouri, and was supplied by Ward’s Natural Science Establishment, Inc. Initially, ground galena was stored in an air tight glass bottle and used for flotation tests after cleaning with distilled water by vigorous shaking and decanting three times. Grinding media was represented by mild steel (AISI 1020), a low erosion and corrosion resistant steel whose presence in grinding circuits is known to adversely affect sulfide mineral flotation. A few tests were conducted with austenitic stainless steel (302), selected for its high corrosion resistance. Magnesium was used to represent an active metal which would not precipitate as a hydroxide under the experimental conditions used in this study.
A JOEL 100 CX scanning transmission electron microscope (STEM) was used to examined the morphology of the surface coatings. Galena samples were first freshly ground or placed in contact with magnesium or mild steel for two hours in oxygenated water and were then dried with methyl alcohol and stored in a vacuum desiccator before being placed in the STEM for photographing.
Results
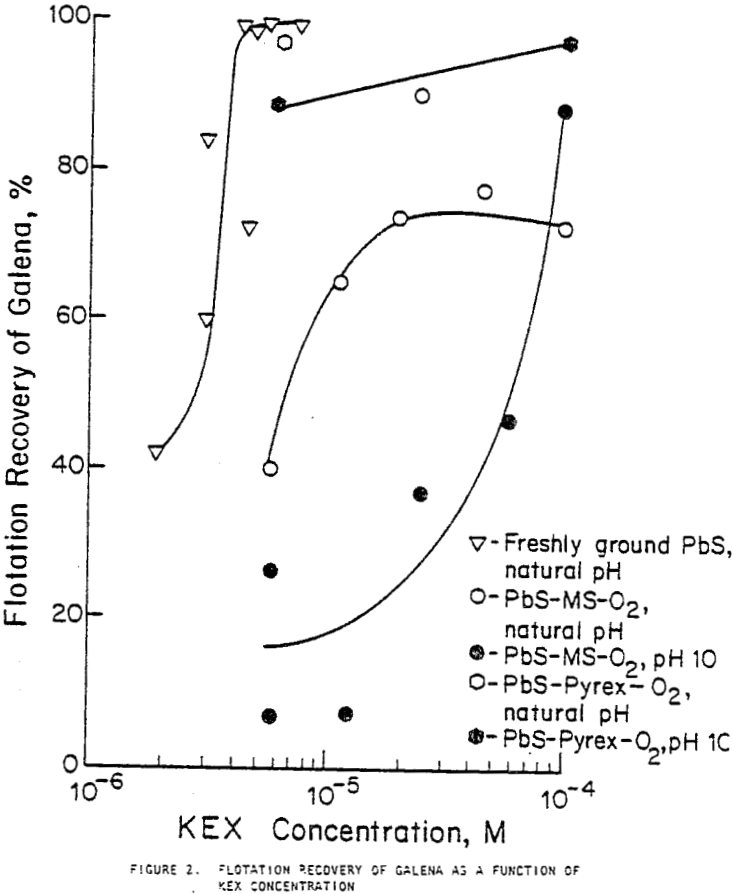
Flotation tests were conducted to determine the effect of galvanic contact of galena with mild steel, magnesium, and austenitic stainless steel. The concentration of the collector, KEX, required to achieve full galena recovery was determined by a series of flotation tests. Recovery at natural, near neutral pH reached 99% with 6 x 10 -6m KEX, the concentration used in subsequent tests.
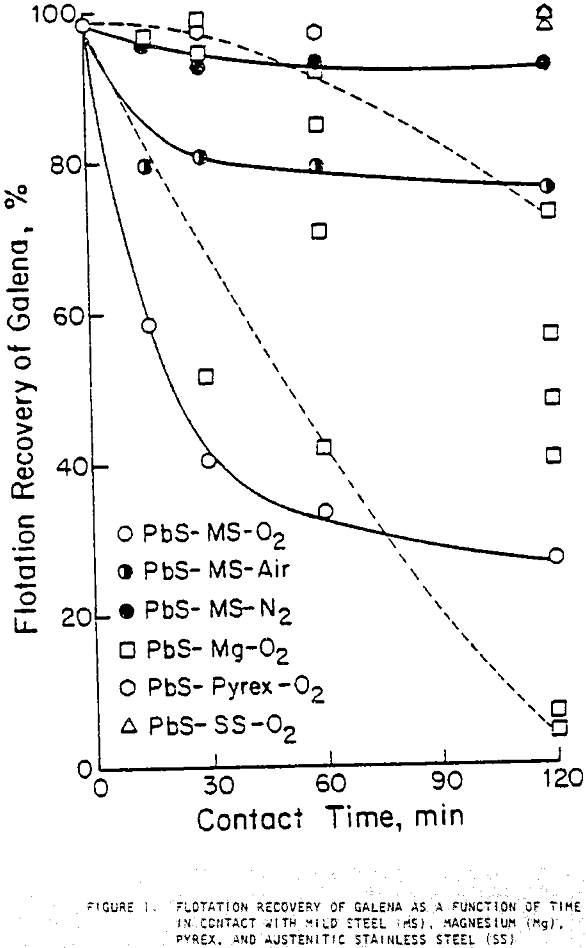
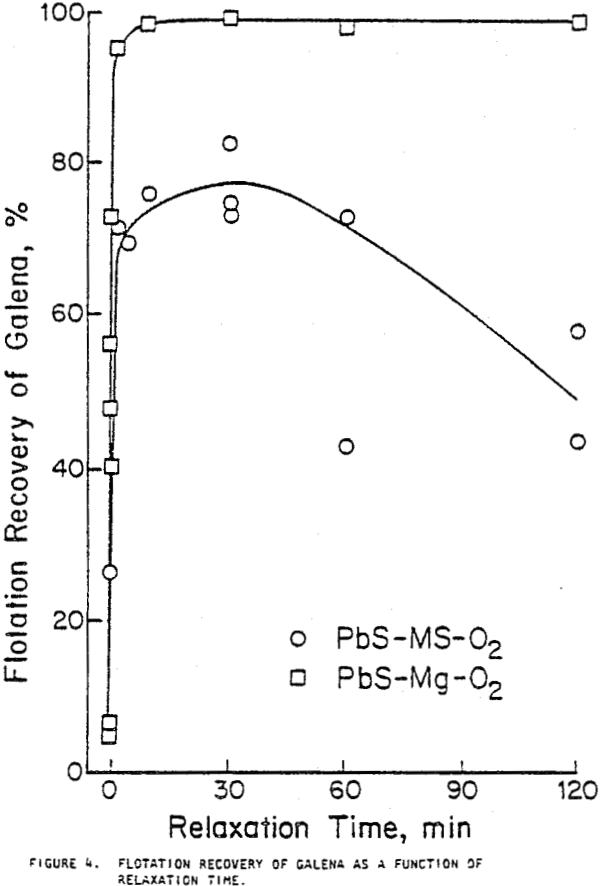
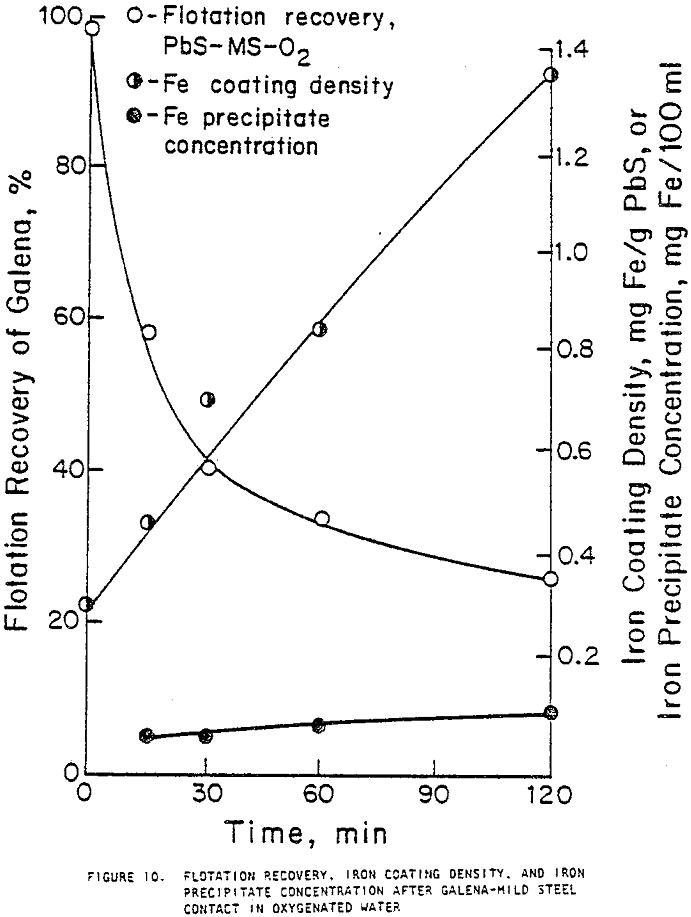
When galena contacted mild steel, its recovery decreased as contact time increased and was lowest with oxygen bubbling and highest with nitrogen bubbling. Contact with austenitic stainless steel and pyrex did not significantly decrease flotation recovery. Contact with magnesium for 30 minutes or less did not substantially decrease recovery, but after one or two hours of contact, large variations in recovery were noted.
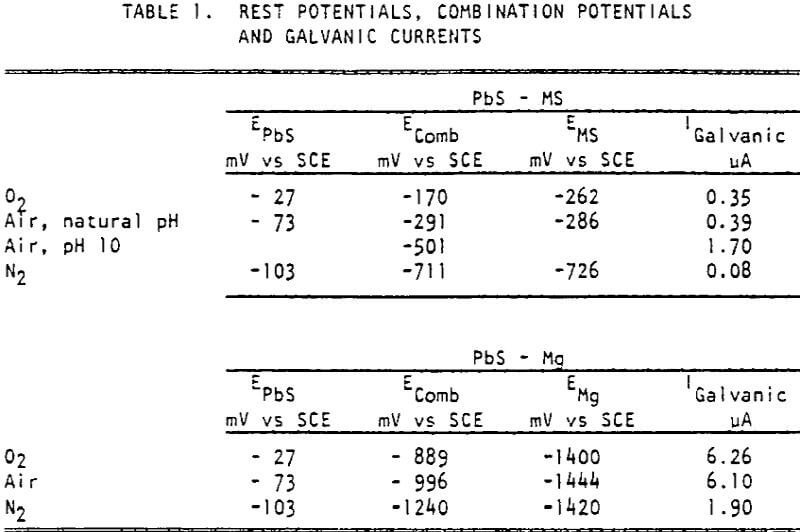
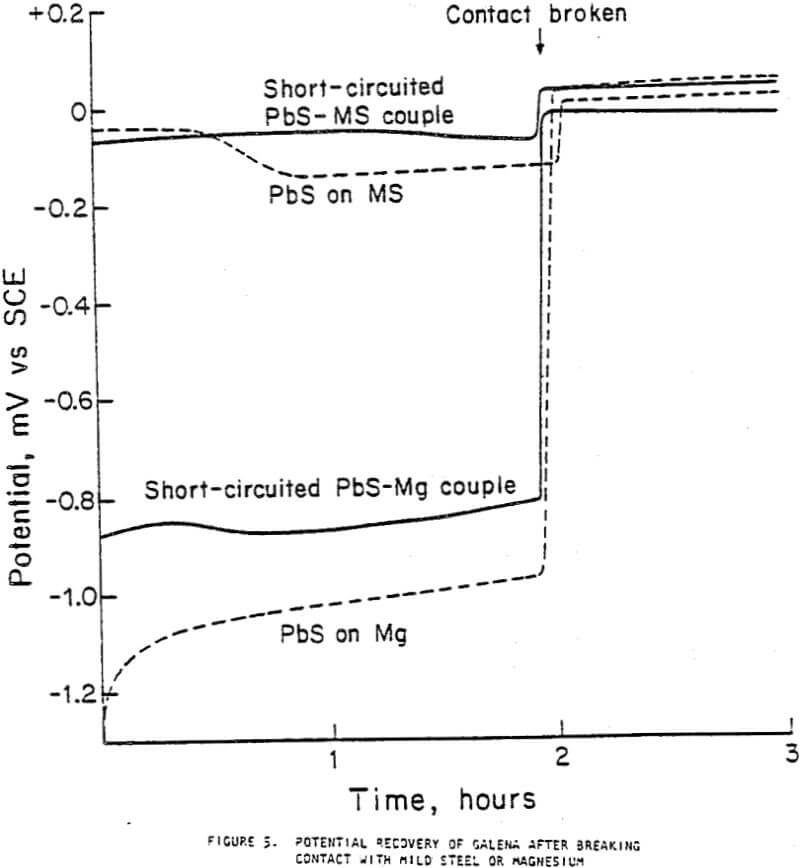
The potentials shifted in the negative direction during the first ten minutes of measurement for mild steel in deoxygenated water and galena in aerated water. Under all other conditions, the potentials shifted in the positive direction. The order of potentials was
PbS > mild steel > Mg
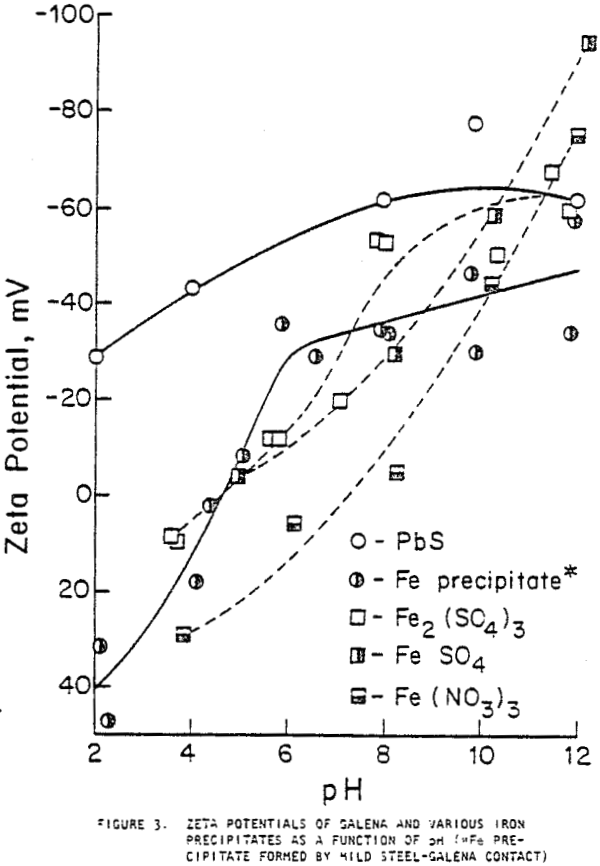
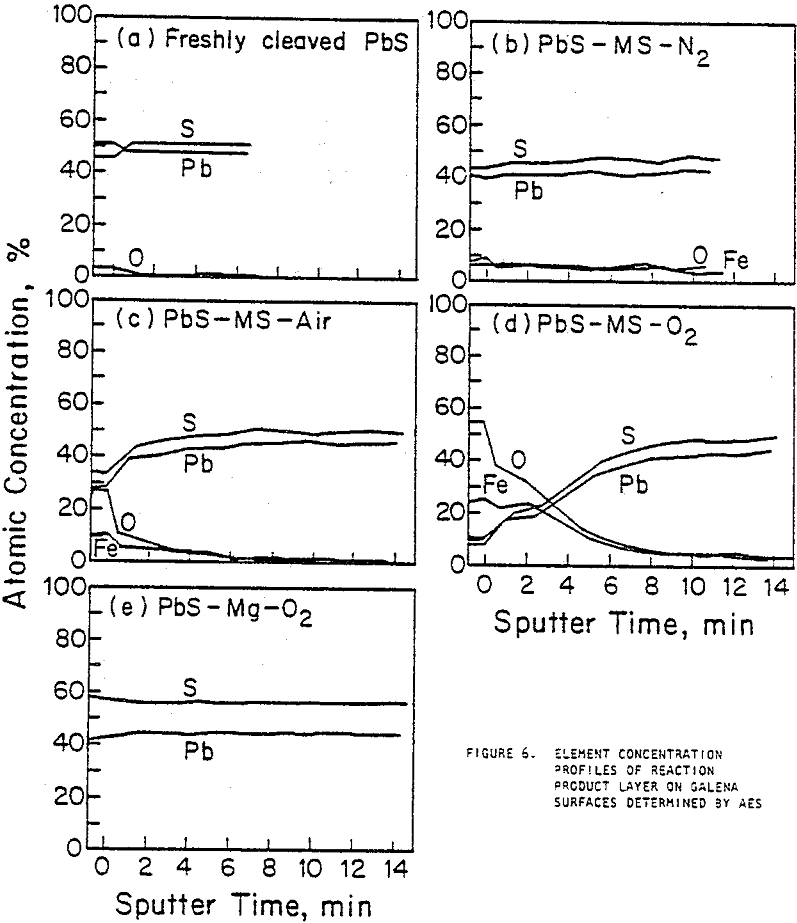
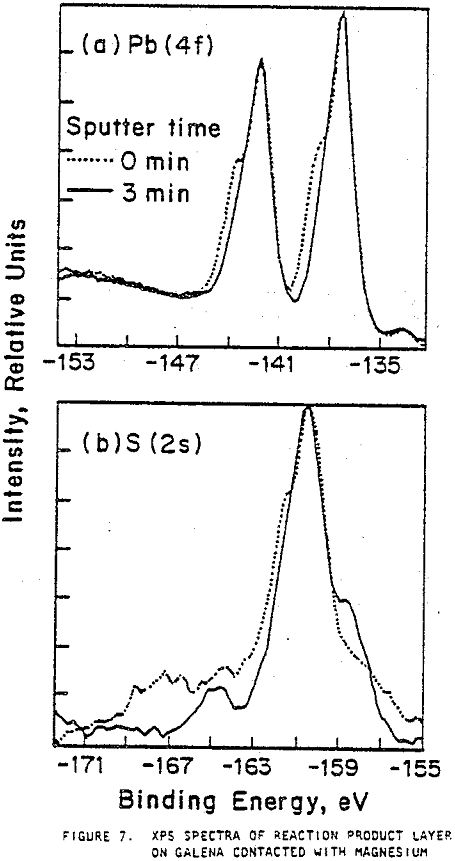
XPS provides information about the oxidation states of elements on the surface and a spectrum of the number of emitted electrons per energy interval versus their binding energy. Spectra were obtained for the major elements on the galena surface before and after three minutes of argon sputtering. The binding energy of lead from galena appeared strongly in all lead spectra with double peaks at about 137 and 142 eV. The sulfur in galena produced a major peak at approximately 161 eV in all the sulfur spectra. The presence of a shoulder on the major lead peaks of galena prior to sputtering indicates the presence of sulfate, sulfite, hydroxide, or carbonate forms of lead. Smaller shoulders were observed after galena contacted pyrex, and larger shoulders appeared after contact with mild steel.
Discussion
The contact of sulfide minerals with grinding media and iron particles abraded from the media during grinding establishes galvanic coupling. The flotation response of galena after contact with mild steel depended both on contact time and the aeration condition of the water in which contact occurred, decreasing with increased contact times and the presence of more oxygen. The rest potentials of galena were more noble than those of mild steel, indicating that they would be galvanically coupled with galena acting as the cathode. Galvanic currents were highest for the galena-mild steel couple in oxygenated water and least for the couple in deoxygenated water, indicating that accelerated electrochemical reactions took place under oxygenated conditions. This correlates strongly with the flotation responses observed.
Galena allowed to relax after two hours of contact with mild steel in oxygenated water showed improved floatability, although full recovery was not achieved as it was when galena was allowed to relax after contact with magnesium. The iron coating density after two hours of contact with mild steel remained high even when the galena was allowed to relax. The inability of galena to achieve full flotation recovery under these conditions may therefore be attributed to the tenacity with which the iron compound coating adhered to the mineral surface.