Table of Contents
Based on the results obtained during this investigation, slag-resistance, open-bath electric smelting of complex lead-zinc sinter is not considered feasible. Inherent characteristics of electric smelting promote and permit excessive transfer of iron, lead, and volatile slag constituents to the condenser when smelting normal lead-zinc sinter. Reaction of these constituents with zinc causes its degradation. Consequently, slag resistance, open-bath smelting is definitely limited to smelting charges which are low in iron and lead content.
Development of an electric smelting process and pilot-scale equipment for sintered, lead-zinc bulk concentrates was undertaken to permit evaluation of any technically successful process to produce lead and zinc simultaneously as distinct products.
Mineralogically complex deposits form a large proportion of known and potential lead-zinc ore reserves. Metals from these resources will be required in the near future to satisfy demand for lead and zinc. However more efficient and more economical methods of exploitation and recovery must be developed if metals from these resources are to be won at a profit. Intimate association of minerals in resources of this character precludes efficient mineral-dressing separations into separate high-grade concentrates suitable for metal recovery by conventional processes. Slag fuming and other retreatment practices have improved metal recovery and smelter flexibility. These scavenger methods do not attack the principal problem, but only recover metals, at additional cost, from products that previously were wasted. In contrast, greater recovery of both metals is usually possible at less cost by bulk smelting of a high-grade, bimetal concentrate containing associated precious metals.
Known domestic resources of complex, lead-zinc minerals are of small to medium tonnage and often are remote from supplies of metallurgical coke. In contrast, those deposits usually are accessible to abundant and cheap electrical power.
Several smelting processes for zinc and for zinc and lead furnish precedents for certain phases of electric smelting of bulk, lead-zinc sinter.
The Trollhattan Electro thermic Zinc process was developed in Sweden to smelt normal high-grade zinc sinter. Research and development of this process extended over a 40-year period, terminating in about 1936. The process employed slag resistance and electric heating, and the final design used a water-cooled, steel condenser to quench zinc vapor to powder. American Cyanamid Company participated in the later stages of process development after many years of work by Swedish and Norwegian groups. Serious difficulties, that were never satisfactorily solved, continually plagued process development; nevertheless, development personnel continued to be enthusiastic. Even after this lengthy period of nearly continuous development, the process was only partially successful. Therefore, experimental development was terminated. It is worthy of note that, in the Trollhattan process, zinc and the small amount of lead in the sinter together with substantial iron were volatilized. Iron, that was transferred to the condenser, combined with zinc and formed a zinc-iron intermetallic in the condensed powder. Zinc recovery from the iron- zinc product constituted one of the major metallurgical and mechanical obstacles.
The Sterling Process for electric-furnace smelting of nonsulfide-zinc ores was essentially a continuing development of the Trollhattan process. The Sterling process employed open-arc open-bath heating rather than slag-resistance heating for the specific objective of reducing zinc oxide and volatilizing prior to fusion of the charge. This smelting and condensing system was developed by New Jersey Zinc Company for treatment of ores containing franklinite, willemite, and zincite. Iron content of the charge was high, and the precious metal content was insignificant. A crude pig iron was produced in the hearth with 6 percent FeO left in the slag. The product of the zinc condenser contained most of the lead and zinc from the charge together with sufficient iron to form appreciable zinc-iron dross. This dross was recirculated to the smelting furnace after liquation. A second Sterling smelting and condensing system was installed at Cerro dePasco’s smelter at La Aroya, Peru. Despite all efforts and major modifications during several years of attempted operation, that zinc smelter was a complete failure and was abandoned in favor of expansion of zinc, electrowinning capacity. New Jersey Zinc Company also has abandoned the Sterling process in favor of the externally heated vertical retort. Although the authors have found no reported explanation for New Jersey Zinc Company abandoning this process, metallurgical and economic factors must have been unsatisfactory. The most significant contribution of the Sterling process to zinc metallurgy was the development of a satisfactory liquid-metal splash condenser. New Jersey Zinc Company continues to use this splash condenser with their externally heated vertical retort, and a modified form of this condenser is employed for several condensing applications.
Duisburger Kupferhuette presently is using an electrothermal method for the reduction, vaporization, and condensation of zinc from zinc oxide clinker obtained from leach residues. The final product is a zinc spelter. This operation is an adaptation of the Sterling process for the electrothermic recovery of zinc from a lead-free, iron-free, and calcined leach residue. A liquid-zinc splash condenser of similar design to the Sterling condenser is employed. Substantial excess coke is maintained in the smelting furnace to provide appropriate electrical conductivity for slag-resistance heating. Byproduct blue powder is sold because recirculation of that product to the smelting furnace proved to be impractical and hazardous. A concentration of FeO in the slag greater than 1.5 percent was found to be decidedly objectionable because iron transfer to the condenser resulted in zinc degradation. No problem with iron exists when smelter feed and fluxes are as iron-free as those used by this German firm. In contrast, smelting of normal zinc sinter by this process would result in a slag high in FeO, which would contaminate an excessive amount of zinc with iron.
The Imperial Process employs blast furnace smelting of bulk lead-zinc sinter and utilizes lead-splash condensing of zinc vapor from the dilute mixture of zinc vapor and smelter gas. This process is metallurgically sound and has gained nearly universal acclaim. Zinc recovery as prime western zinc is in excess of 90 percent. Lead bullion recovery, containing precious metals, is about 94 percent. The National Smelting Company has spent nearly 30 years in research and development of this lead-zinc, blast furnace and lead-splash, zinc condenser. Eight of the world’s newest smelters employ this process. The success of this modern process is attested by this general acceptance. This process accomplishes efficient recovery of metal from simple zinc sinter or from bulk zinc-lead sinter. Because lead oxide reduction is exothermic, lead production by this process imposes no thermal burden on the blast furnace. An average of 0.024 percent iron reports in zinc spelter produced by the Imperial process compared to approximately 2.0 percent in zinc produced by electric-furnace smelting. Several factors contribute to this lower iron content of blast furnace zinc spelter. Maximum temperatures are much lower in the blast furnace than in furnaces using electric-arc or slag-resistance heating. The blast furnace has a dry-charge column containing coke that filters much of the smelter dust and fume from the mixture of zinc vapor and furnace gas. Zinc vapor-gas mixture emerges from the dry-charge column of the blast furnace at about 110° C. This vapor-gas mixture is elevated to a temperature above 1,000° C prior to entering the condenser to prevent oxidation of zinc. This increase in temperature is attained by carefully controlled induction of air above the charge column to burn some CO to CO2.
Much effort has been devoted to developing electric-smelting methods for zinc, and the production of lead and zinc as distinct products by smelting roasted lead-zinc concentrate is not a new idea. The Imperial process is a success; however, certain advantages of lead-zinc electric smelting appeared attractive if an efficient process and satisfactory equipment were developed. Since electric smelting could prove efficient in units of less capacity than that found practical for blast furnaces, electric smelting might have certain advantages for mines and districts of medium reserves of complex ores. Electric smelting could have a cost advantage in areas of abundant and low-cost electrical power, transportation difficulties, and costly coke. Therefore, because of the possible contribution toward profitable exploitation of submarginal, complex lead-zinc resources, lead-zinc electric smelting was thoroughly investigated.
Theoretical Considerations
Electric smelting of complex lead-zinc sinter encompasses two essential considerations. Reduction and condensation thermodynamics and kinetics must be understood and these principles must be applied.
The theoretical metallurgy of zinc has been well described in the literature. Smelting reactions encountered in combined lead-zinc sinter are complicated by reduction of lead and iron oxides. Reduction reactions may occur at the solid-gas interface, the liquid-gas interface, or by direct reduction in the liquid slag. Since this is a nonequilibrium, heterogeneous system, equilibrium data and calculations are limited to define the maximum conditions under which the reduction reactions may proceed. Reduction reactions are described by kinetic factors such as activation energy and absolute reaction rates. Since these properties describe reaction progress, they must be obtained experimentally and cannot be obtained from thermodynamic data.
The principal solid-gas reduction reactions and their heats of reaction and free energy at 1,600° K are :
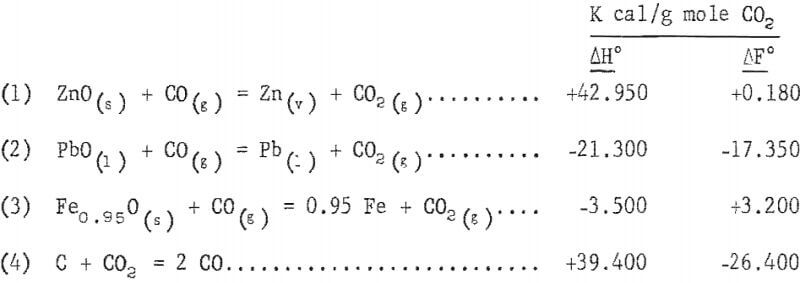
Reactions 1 and 4 are endothermic, and reactions 2 and 3 are exothermic. The overall energy requirement is endothermic.
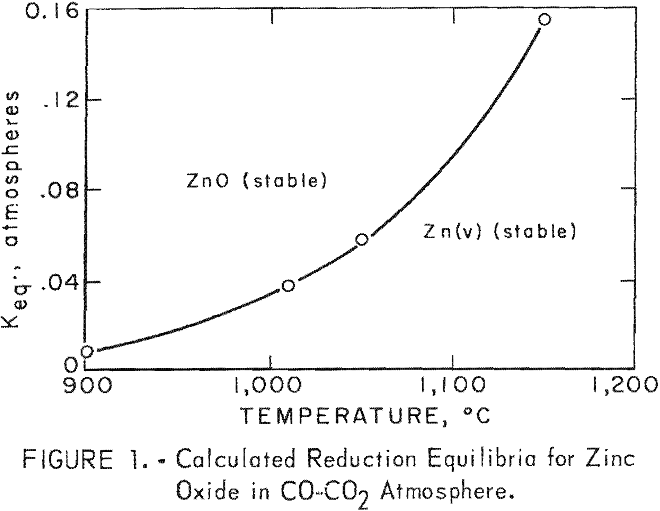
Meaningful equilibrium calculations can be made in the gas-zinc vapor stream. The gas-zinc vapor can be considered uniform in temperature and composition, and the kinetics of reaction are sufficiently rapid to achieve equilibrium during passage from the furnace to the condenser. Defining and isolating the system in this manner permits a rigorous thermodynamic evaluation. The equilibrium temperature below which reoxidation occurs for a specific mixture of zinc, vapor and reduction gas is calculated according to the following chemical reaction and free-energy relationship:
ZnO(s) + CO(g) = Zn(v) + CO2(g)
ΔF° = 42,640 – 26.64 T = -4.58 T log Keq………………………………..(2)
T = degree Kelvin
Keq = (pZn) (pco2)/(pco)
Keq = equilibrium constant
p = partial pressure
A plot of Keq versus temperature is shown in figure 1. This figure shows the importance of maintaining a specific mixture of zinc vapor and reduction gas above its equilibrium temperature. A mixture of zinc vapor and reduction gas, having a volume-percent composition of 40 zinc vapor, 45 CO, 7 H2, 6 CO2, and 2 N2 (approximate analyses of mixtures of zinc vapor and reduction gas obtained in this investigation), must be maintained in excess of 1,054° C to prevent reoxidation.
In addition to the thermodynamic aspect, the kinetics of mechanisms of zinc vapor condensation must be considered. Zinc vapor can condense on the condenser walls, on drops of zinc, on the metal bath in the bottom of the condenser, or on dust particles in the gas stream. Zinc which condenses on a surface of molten zinc will become part of the molten mass and will be recovered as liquid zinc.
Zinc which deposits on a dust particle nucleus in the gas stream will form a droplet which may or may not coalesce with the molten bath. Two factors may interfere with coalescence. First, carbon dioxide will oxidize the surface of the droplet and this oxide film will interfere with coalescence. Second, cooling of the droplet by radiation from the cooler walls of the condenser or by giving up heat to other elements of the gas stream may result in solidification of the particle and prevent its coalescence with the metal bath. Under drastic cooling conditions, more nuclei become condensation centers and fine particles of “physical blue powder” are formed. Partially oxidized physical blue powder will not coalesce with the metal bath.
Application of basic thermodynamic and kinetic, metallurgical concepts is vital to zinc condensation and coalescence.
Sintering Experiments
Equipment and Materials
Pilot-plant, updraft, sintering equipment was designed and fabricated as shown in figure 2. The cylindrical sinter pots were 12 inches high and had a grate area of 5 square feet. Air flow through these sinter pots was maintained by a high-velocity suction fan controlled by two dampers. Provision was made in the side of the sinter pot for insertion of three thermocouples spaced at 2-, 4-, and 6-inch level. A thermocouple was placed in the exhaust flue.

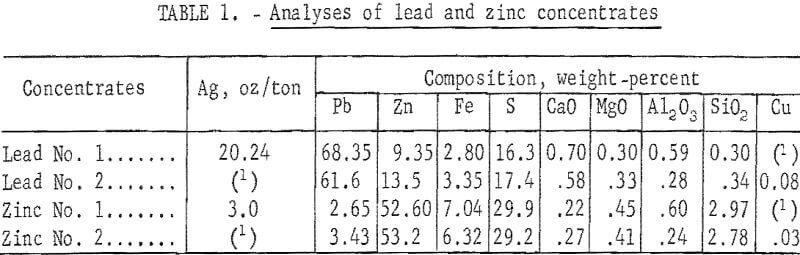
Material used for sinter preparation consisted of a blend of selective lead and zinc concentrates. Analyses of these concentrates are presented in table 1.
Procedure
Blended lead and zinc concentrates were pelletized to a minus ½-inch product. Four cycles with intermediate grinding and repelletizing were required to obtain satisfactory desulfurization. Temperatures of the sintering zones were controlled by manual adjustments of the draft.
Results
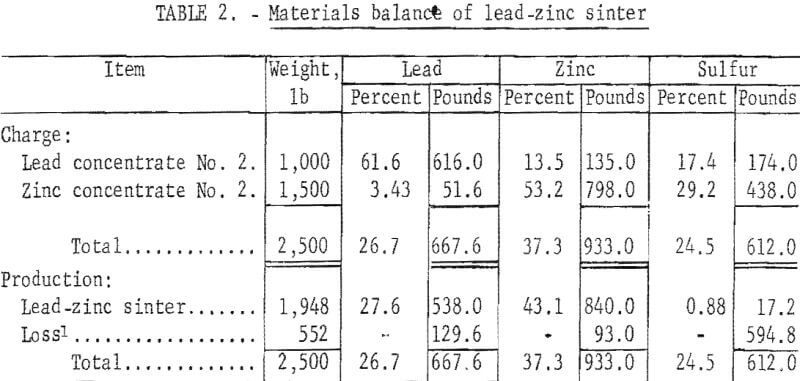
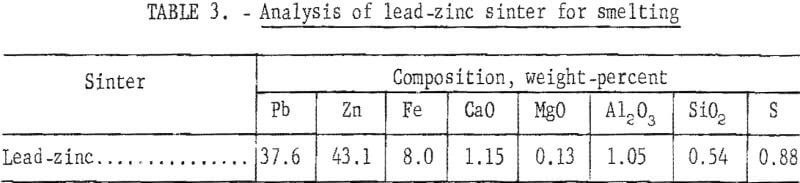
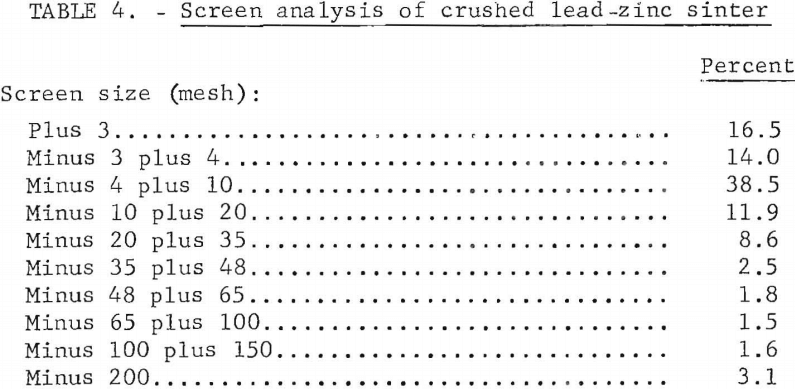
A partial materials balance, product analysis, and a screen analysis of typical crushed sinter are shown in tables 2,3, and 4, respectively.
Discussion
Initial sulfur content of 25 percent in combined lead-zinc concentrate was progressively decreased to less than 1 percent in the final sinter. Elimination of 97.2 percent of the initial sulfur was considered satisfactory. Lead and zinc losses of 19.4 percent and 10.0 percent, respectively, would be reduced in conventional practice. Although updraft sintering desulfurizes effectively, current practice of excessive recycling of the sinter indicates a need for more efficient desulfurization.
A microprobe study and analysis, conducted by P. A. Romans, revealed the complex association of the sinter constituents. A sample of that sinter contained at least six phases. Identified phases with approximate composition and probable order of prevalence in the sinter are summarized in table 5. Chemical analysis of the sinter sample used for microprobe analysis is presented in table 6.
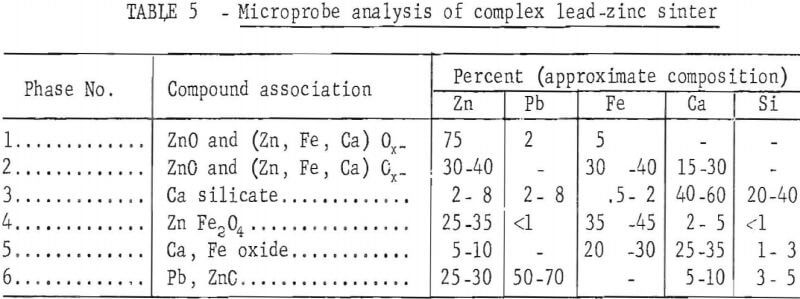
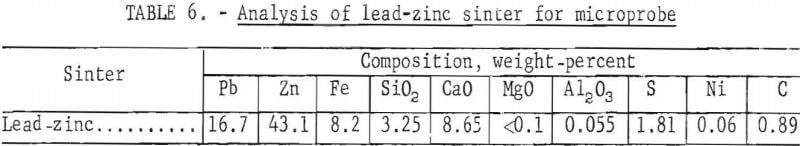
A description of the identified phases indicates phase 1, the predominating dendritic phase, is probably a suboxide; phase 2 is the matrix for phase 1 and probably a suboxide; phase 3 is probably a calcium silicate; phase 4 may be ZnFe2O4; phase 5 is probably calcium iron oxide; and phase 6 may be metallic lead, but is more likely a suboxide with particle size 2 to 5 microns. Sulfur, is present, exists as a minor part of any phase. As a result of non-equilibrium conditions during formation, there is substantial composition variation from one part of a phase to another.
Smelting and Refining Investigations
Smelting Equipment System and Materials
Several electric-furnace types were used for initial tests. Following several unsuccessful attempts with these experimental furnaces, a small pilot-scale unit was designed and fabricated. Many minor modifications were made to improve this design. Figure 3 shows a front sectional view of the electric-smelting furnace. A magnesite furnace refractory was used for most of the experiments to provide the basic lining appropriate for the corrosive slags high in iron oxides from bulk lead-zinc sinter. A carbon-block refractory was used for some special experiments in which iron-free charges and slags were employed.
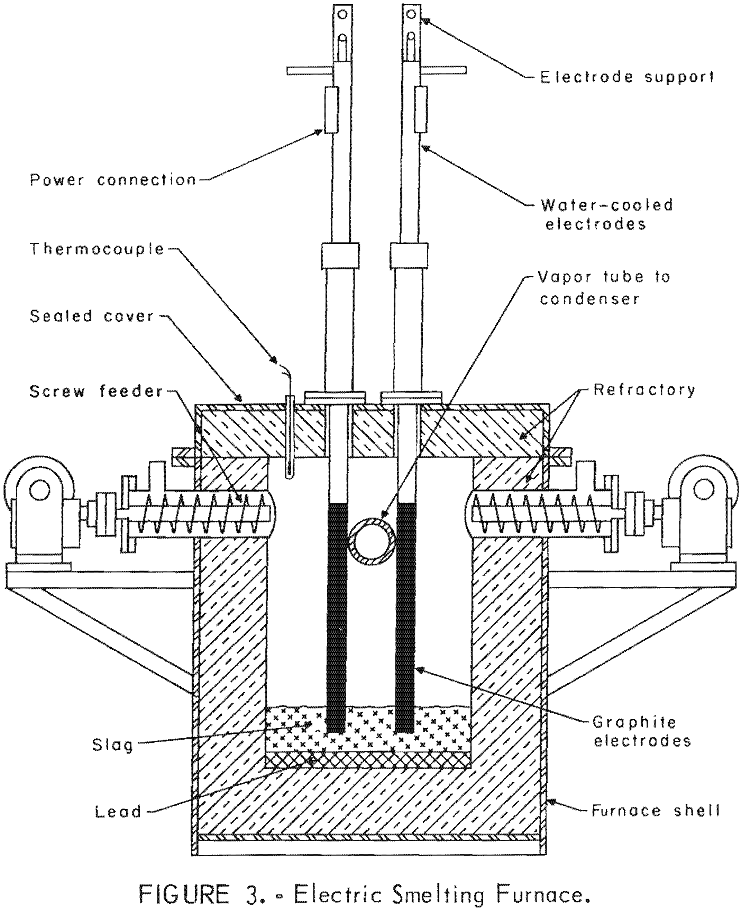
Two condensing systems were designed. Initial closed -system smelting and condensing employed a water-cooled copper – jacketed condenser connected to the furnace discharge flue. That condenser was designed to quench the mixture of zinc vapor and reduction gas from approximately 1,000° to 25° C. This shock condensation produced zinc and lead as pyrophoric powder. Carbon monoxide and carbon dioxide were the major constituents of the exhaust gas. A settling box and fiberglass filter system were designed to remove the remaining fine powder from condenser exhaust gases. A sealed cleanout system was provided to permit periodic cleaning of the connecting flue between furnace and condenser.
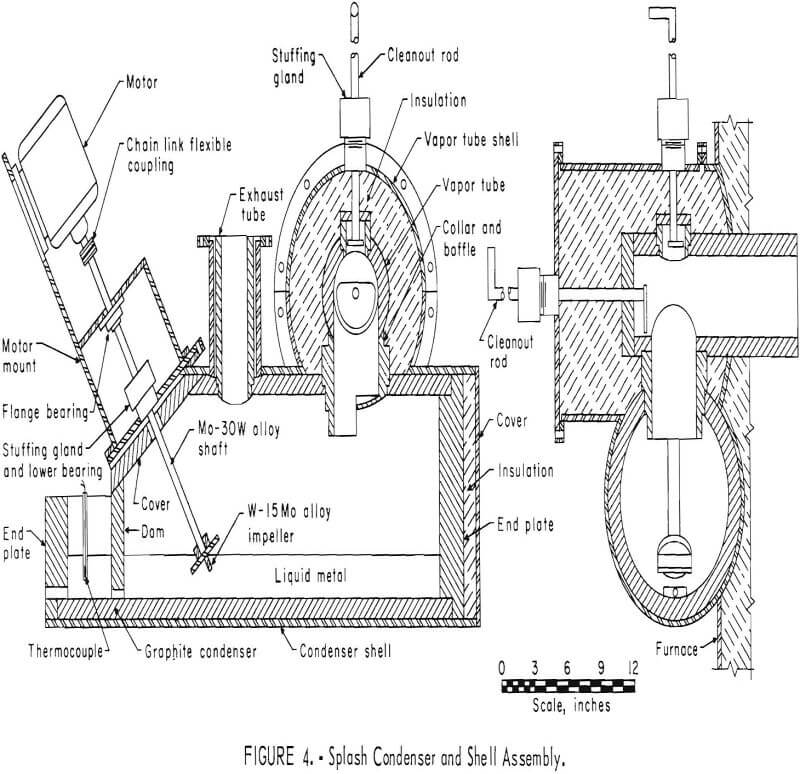
Subsequent smelting experiments employed a molten metal-splash condenser of similar design to that developed by New Jersey Zinc Company. This pilot-scale condenser was installed to permit production of metallic zinc on a continuous basis. Selection of construction materials required substantial experimentation. A graphite-lined condenser proved satisfactory. Calrod elements furnished initial heating and heat control of the condenser and its splash medium. Zinc vapor was effectively condensed by the molten-metal splash obtained by rotating a 3-inch-diameter impeller 1,740 rpm. Tungsten-molybdenum alloys possessed the physical properties required for this service. A molybdenum-30 percent tungsten impeller shaft was at 38° from vertical. The tungsten-15 percent molybdenum impeller was about two-thirds immersed in molten metal. Blue powder and gas leave the condenser at a temperature of 500° C, but they are cooled to approximately 100° C before discharging freely from the stack. Figure 4 shows this splash condenser design. Figure 5 is a photograph of the assembled smelting furnace and condensing system in operation.
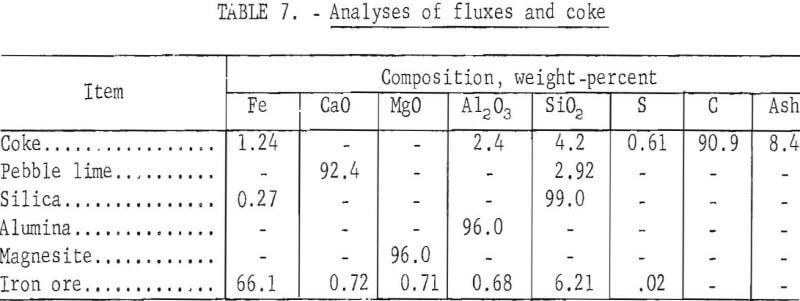
Analyses of flux materials and coke are presented in table 7.
Procedure
Certain conditions were found essential for smelting complex lead-zinc sinter. A slag was required that was fluid and had appropriate electrical resistivity. Minimum slag-fall and minimum refractory corrosion were essential. For a specific mixture of zinc vapor and CO-CO2 gas, a temperature in excess of the equilibrium temperature must be maintained to prevent oxidation of zinc vapor.
Smelting tests were designed to establish optimum parameters. Smelting parameters were slag composition, reduction requirements, temperature, gas composition, and refractory performance.
A propane burner was used to preheat the furnace. Approximately 100 pounds of lead was charged and melted. A synthetic slag mixture was charged. The furnace top was assembled. Cooling water and nitrogen purge for the

electrode stingers were adjusted. The ventilation system was connected to the furnace and condenser. Power was turned on the furnace, and the electrodes were adjusted to obtain slag-resistance heating. Additional slag mixture was charged, and the furnace was heated to a top temperature of approximately 1,450° C. Gases and fumes, generated during this heating period, were bypassed to the dust collector to avoid oxide accumulation in the metal-splash condenser. When a satisfactory operating temperature had been obtained, the furnace exhaust gas was directed through the condenser. A uniform charge rate of approximately 45 pounds per hour was established and maintained. Power was adjusted to supply the necessary thermal requirements for the endothermic zinc oxide reduction and to maintain the gas-zinc vapor mixture above the equilibrium temperature. Condenser exhaust-gas samples were taken during tests. A slight, positive pressure of reaction products was maintained to prevent aspiration of air into the system. This positive pressure prevented zinc reoxidation and hazardous explosions of the mixture of zinc vapor and reduction gas in the furnace and condenser. Reduction was continued for an additional 15 minutes after sinter charging was terminated. Slag and lead were tapped, and the power was shut off. Metal was drained from the splash condenser. Furnace and condenser were disassembled, and the equipment was inspected. Products were sampled and analyzed, and a material balance was prepared for completed tests.
Results of Water-Cooled, Condenser Tests
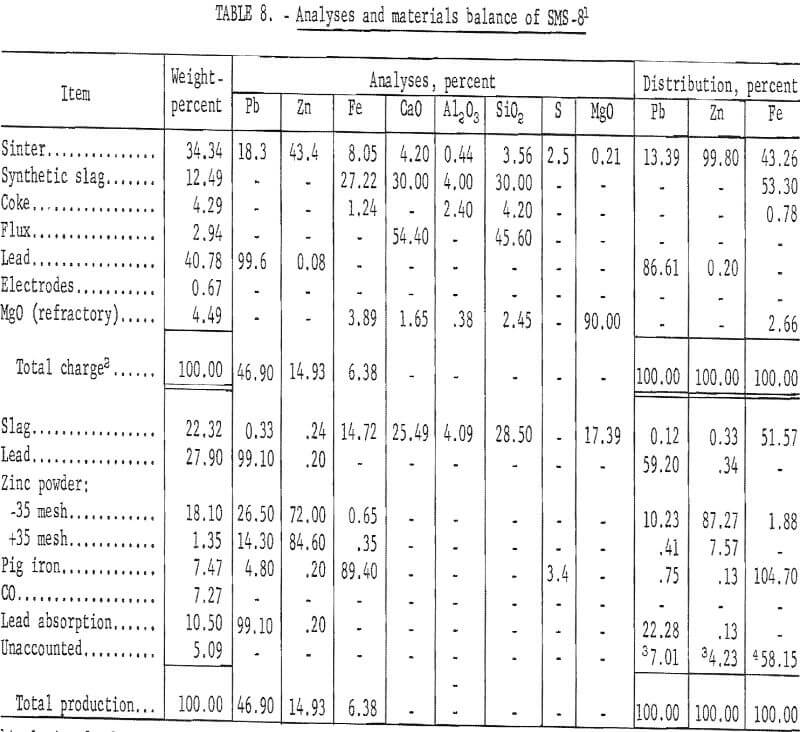
Eight smelting tests were conducted utilizing the water-cooled condenser. Quantitative results of a typical smelting test, which produced pyrophoric powder, are presented in table 8.
All smelting tests during this phase of investigation indicated excellent lead and zinc elimination from the slag. Lead and zinc metal accountability exceeded 90 percent. Lead volatilization to the condenser was excessive. Some iron was transferred to the condenser, and some offgrade pig iron was produced in the furnace.
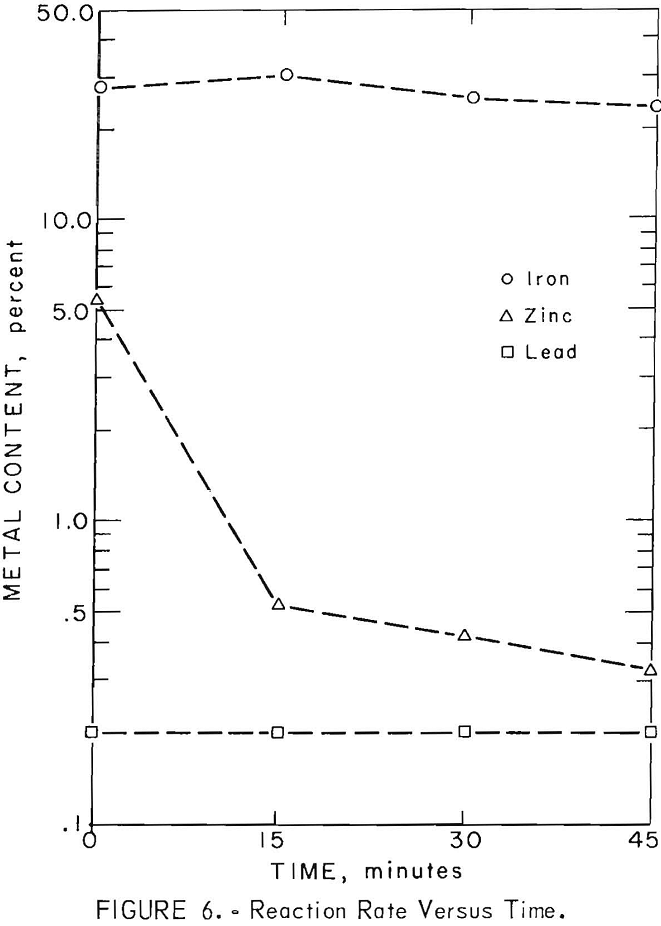
Reduction rates of lead, zinc, and iron in the slag were determined during the seventh smelting test. “Cold -bar” slag samples were taken at 15 minute intervals after sinter charging was completed. The rate, of lead, zinc, and iron reduction in the slag is illustrated in figure 6. Lead and zinc were eliminated to 0.2 and 0.5 percent respectively during the first 15 minute interval. Iron reduction became significant after most of the lead and zinc had been eliminated.
Lead and zinc recoveries, as pyrophoric powder, were considered successful. Slag composition, coke requirements, and approximate operating temperatures were established for the pilot-plant smelting and condensing system.
Results of Molten-Metal, Splash-Condenser Tests
Based on the encouraging results obtained from the water-cooled condensing experiments, a molten-metal splash-condensing system was designed and fabricated to permit investigation of continuous production of zinc spelter.
Smelting procedure was essentially the same as that employed in previous tests. Approximately 150 pounds of molten lead were charged to the electrically heated splash condenser. The temperature of the splash medium was maintained at approximately 500° C. The importance of maintaining the gas and zinc vapor stream above equilibrium temperature became quite apparent after a series of metallurgical failures. Excessive oxidation of zinc vapor occurred despite numerous attempts to lower CO2 content. Not only were these tests metallurgical failures, but many dangerous situations developed because obstructions in the exhaust duct pressurized the furnace.
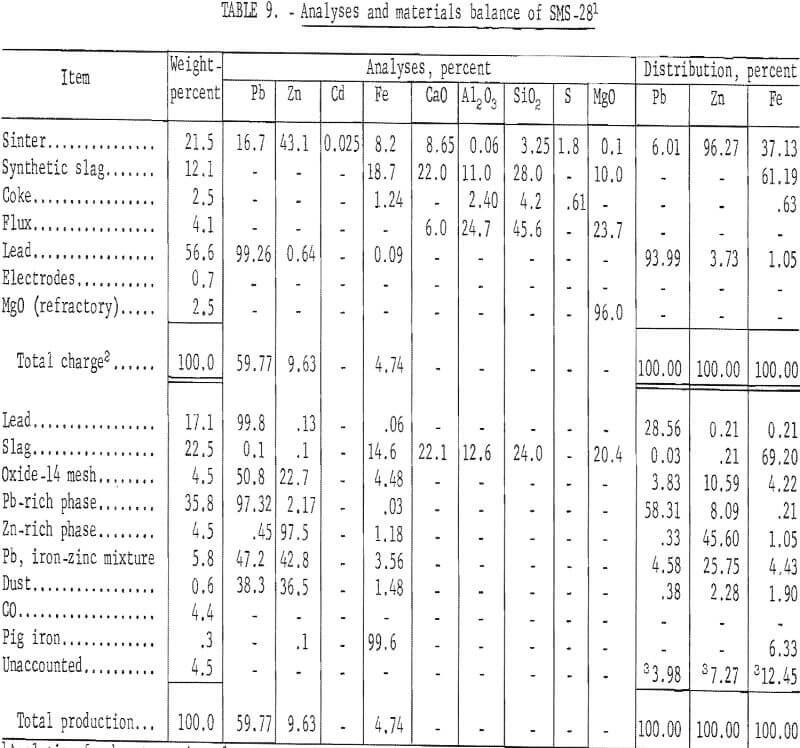
The first partial success in recovering zinc spelter was achieved by employing an externally heated passage between the furnace and condenser. The Nichrome and Kanthal heating elements failed. Consequently, a more practical means was required to resolve the problem of heat transfer. A short-coupled connection from furnace to condenser provided the thermal requirements by minimizing heat losses. As a result of these modifications, zinc recovery was partially successful. Quantitative results of a typical test are presented in table 9.
Zinc metal recovery was 53.9 percent. An iron-zinc intermetallic compound contained 25.75 percent of the zinc.
The problem of chemical blue powder formation was alleviated by providing an adequate temperature for the mixture of gas and zinc vapor; however, two other major problems affecting zinc metal recovery were identified. Volatile slag and metal constituents were transferred from furnace to condenser and reacted with zinc vapor and liquid zinc. An iron-zinc intermetallic compound and oxides were produced.
X-ray identification of condenser metal oxide byproduct revealed the primary constituent as PbSO4 and the secondary constituent as zinc ferrite (ZnO·Fe2O3). A microprobe study and analysis were made of this mixture of lead and an iron-zinc intermetallic compound. Microprobe analysis revealed the specimen to be a mixture of many phases. Zinc and iron-zinc alloys, lead and lead oxide, zinc sulfide, and zinc oxide in descending order constitute roughly 95 percent of the specimen. The specimen can be divided into three regions with increasing lead and decreasing zinc content. The first region contains lead particles surrounded by a mixture of FeZn10 and zinc. Zinc metal, in this region, contains about 0.9 percent iron and the FeZn10 about 8.5 percent iron. Under the optical microscope, the zinc metal is light compared with FeZn10 and occupies about 20 percent of the region’s volume. Lead grains appear to have trapped ZnS particles which contain less than 0.5 per-cent iron and a maximum of 10 percent lead. Large dark voids have appreciable ZnO at the bottom. Small quantities of calcium, iron, and zinc compounds are also present. A second region has a lead matrix surrounding well developed FeZn10 grains, typically 20 to 60 microns across. This is essentially a two phase region; however, the lead matrix contains some ZnS and the FeZn10 grains have a high iron core. The third region contains substantial lead, Fe3Zn10 having a high iron core, ZnS, and extensive voids partially filled with broken chunks of ZnO. Smooth lead oxide surrounds many of the pores and voids. Lead oxide is present in the lead matrix. Other compounds containing calcium, iron, and zinc (probably as oxides of varying composition) are also found in this region.
Smelting of complex lead-zinc sinter and condensation of lead and zinc metal proved to be more difficult than the previous phase of investigation where pyrophoric lead and zinc were produced. Problems encountered were both mechanical and metallurgical. Special tungsten-molybdenum alloys were required for the splash impeller and shaft. Other materials, such as a D-36 (Cb-Ti-Zr) alloy and 316 stainless steel protected by graphite, were inadequate for this service. Metallurgical requirements imposed stringent demands on the condenser system design and construction materials. Some of the metallurgical demands were met, and partial success in zinc metal recovery was achieved; however, attempts to improve zinc metal recovery to acceptable standards were unsuccessful. Evaluation of the data obtained from smelting tests indicated that the principal factor which caused unsatisfactory yields of zinc metal was the degradation of zinc to an iron-zinc intermetallic compound. Special tests were designed to differentiate the effects of several smelting parameters on zinc metal recoveries.
Special Smelting Investigation
Special smelting tests were conducted to determine the cause of unsatisfactory zinc metal recovery obtained by smelting complex lead-zinc sinter in the pilot-plant smelting and condensing equipment.
Conventional electrothermic smelting of zinc oxide, the effect of zinc ferrite, the effect of lead oxide, and the combined effects of zinc ferrite and lead oxide were investigated. Molten lead was utilized as the condenser splash medium. Equipment and operating procedure were essentially equivalent to those employed during smelting of complex lead-zinc sinter. Installation of a carbon-block furnace refractory and arcing on a coke bed were the exceptions. Reagent-grade zinc oxide, lead oxide, and high-purity iron powder were utilized for charge preparation.
Conventional Zinc Oxide Smelting
Procedure and Materials
Pelletized reagent-grade zinc oxide was fluxed to produce a final slag having a nominal composition of 29.2 percent CaO, 14.6 percent MgO, 14.6 percent Al2O3, and 41.6 percent SiO2. A layer of lead was used on the furnace hearth. A layer of coke floating on the surface of the slag was essential to permit development of sufficient energy.
Results
Analyses and materials balance of this test are presented in table 10.
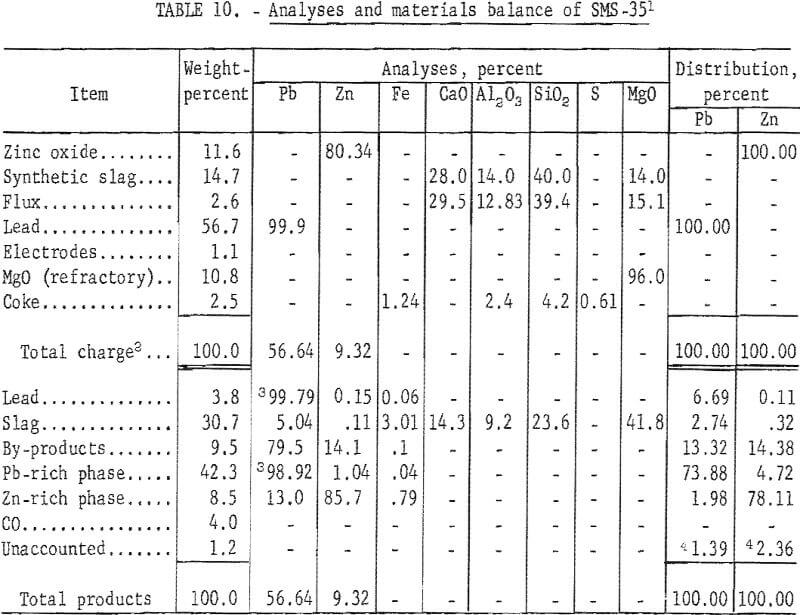
Evaluation of the results shows a zinc recovery in metal products of 82.94 percent. Byproduct zinc from the condenser and baghouse accounted for 14.38 per-cent of the zinc charged. Zinc losses in the slag were 0.32 percent. Lead accountability was 98.61 percent. Excessive volatilization of lead from the furnace to the condenser can be attributed to the localized high temperatures obtained in electric-furnace smelting. Recovery of zinc metal was approximately 83 percent. These recoveries are comparable to those obtained by conventional pyrometallurgical processes.
Effect of Iron (Zinc Ferrite) on Recovery of Zinc Metal
Procedure and Materials
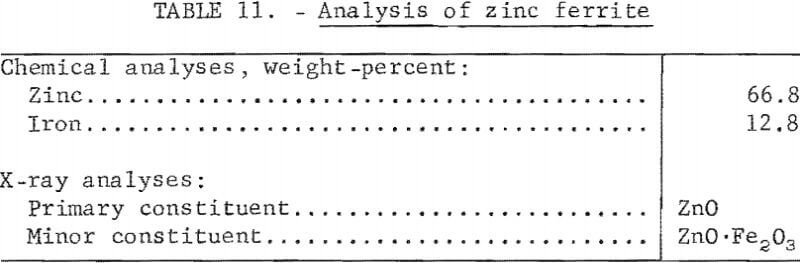
Reagent-grade zinc, oxide and iron powder were blended and treated in an electrically heated kiln at 950° C to produce zinc ferrite. The iron to zinc ratio was 0.192 to 1, equivalent to that of complex lead-zinc sinter used in previous smelting tests. X-ray identification of the synthetically prepared zinc ferrite confirmed complete ferritization (analysis shown in table 11).
Pelletized zinc ferrite was fluxed to produce a slag having a nominal composition of 24.1 percent FeO, 22.0 percent CaO, 10.0 percent MgO, 11.0 percent Al2O3 , and 28.1 percent SiO2.
Results
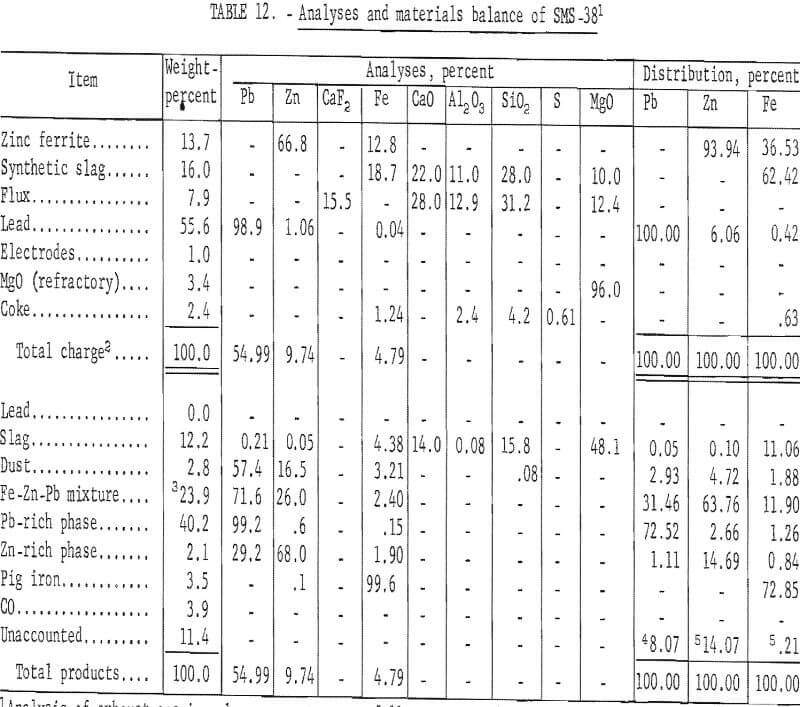
Analyses and materials balance of a typical test are presented in table 12.
Evaluation of these data shows 17.35 percent of the zinc was recovered as metal. Condenser and baghouse byproducts accounted for 68.48 percent of the zinc. The principal zinc-bearing byproduct was an iron-zinc intermetallic compound in a lead matrix.
Effect of Lead Oxide on Recovery of Zinc Metal
Procedure and Materials
A mixture of reagent-grade lead oxide and zinc oxide, having a lead to zinc ratio of 0.68 to 1, was prepared for this smelting charge. This ratio was selected because it is approximately equal to that of complex lead-zinc sinter used in previous smelting tests. Coke was added to provide approximately 130 percent of stoichiometric carbon required for zinc oxide reduction. Flux was added to produce a slag having a composition of 41 percent CaO, 15 percent MgO, and 44 percent SiO2. Carbon-block refractory was used, and a coke bed replaced the usual lead on the hearth.
Results
Table 13 presents the results obtained by smelting a charge containing zinc oxide and lead oxide.
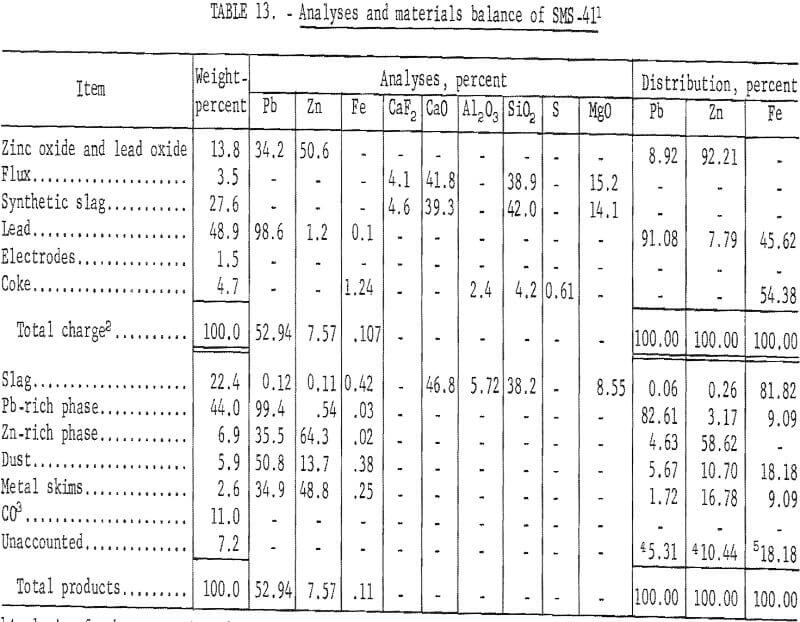
These results show a 61.82 percent recovery of zinc metal. Condenser byproducts accounted for 27.48 percent of the zinc charged. All of the lead from the smelting charge was vaporized and transferred to the condenser and baghouse.
Combined Effects of Lead Oxide and Zinc Ferrite on the Recovery of Zinc Metal
Procedure and Materials
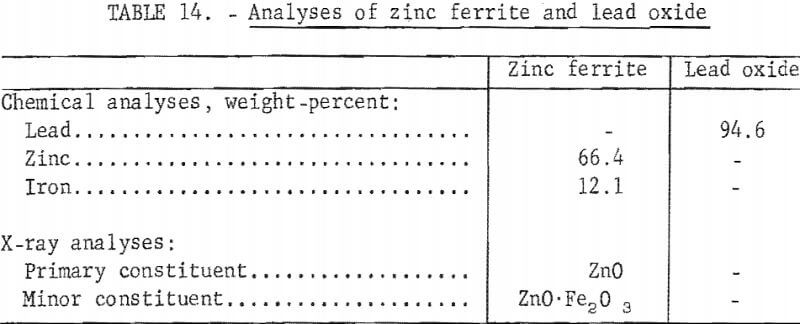
Analyses of synthetically prepared zinc ferrite and reagent-grade lead oxide are presented in table 14. The iron- and lead-to-zinc ratios of the blended charge were 0.18 and 0.64 respectively. These ratios approximate those in the complex lead-zinc sinter used in previous smelting tests. Flux was added to produce a slag having a composition of 24 percent FeO, 22 percent CaO, 10 percent MgO, 11 percent Al2O3, 28 percent SiO2, and 5 percent CaF2. Coke was added to provide approximately 130 percent of stoichiometric carbon required for zinc oxide reduction.
Results
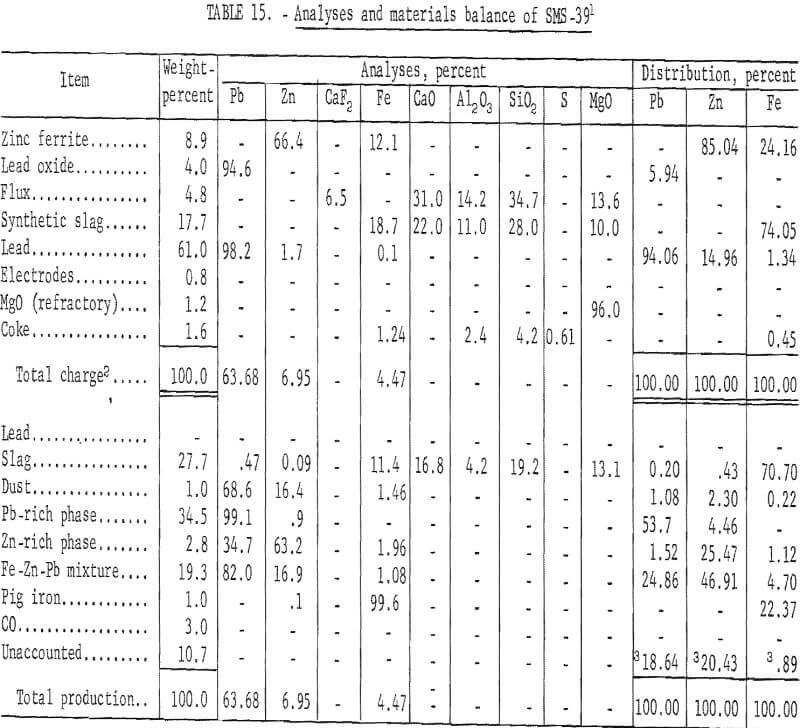
Table 15 presents results of this phase of investigation to determine the combined effects of zinc ferrite and lead oxide on recovery of zinc metal.
These results show 29.93 percent of the zinc recovered as metallic zinc and 49.21 percent in condenser and baghouse byproducts. The condenser zinc-rich phase contained 1.96 percent iron and would require refining to produce salable zinc metal.
Refining Investigation
A special investigation was conducted at College Park Metallurgy Research Center to determine the amenability of the iron-zinc intermetallic compound to refining techniques by centrifugation or filtration.
Procedure and Materials
The procedure entails precipitation of iron with aluminum to form the compound Fe2Al5. The molten lead-rich phase was separated by gravity, and the zinc-rich phase was separated from the iron-aluminum compound by filtration. A description of the filtration process appears in a Bureau of Mines publication.
Results
Analyses and materials balances of this refining investigation appear in tables 16 and 17.
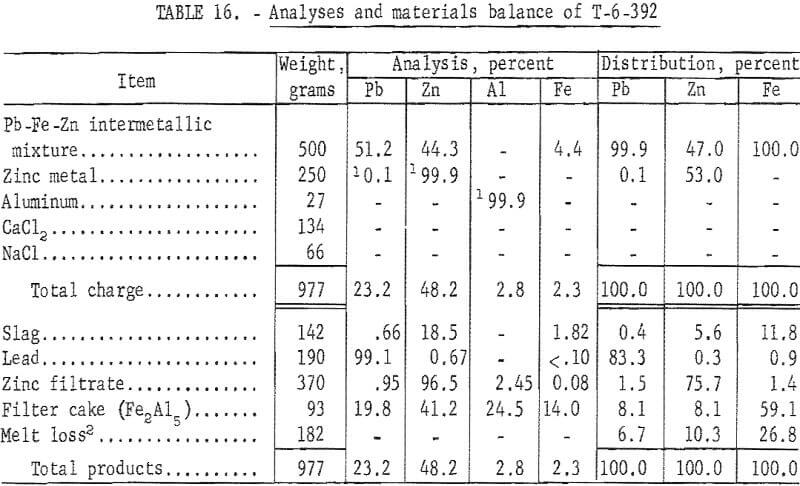
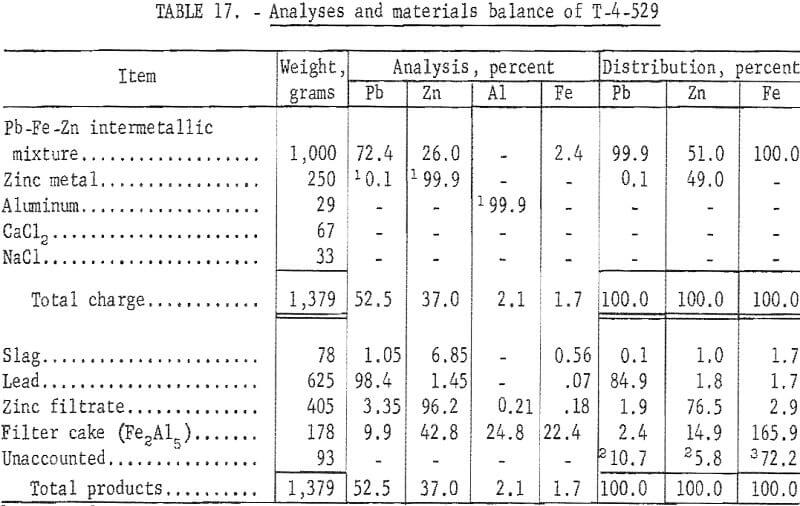
The iron-zinc product, T-392, was a byproduct from SMS -27 smelting test on lead-zinc sinter, and T-529 was a similar byproduct from SMS-38 smelting test on synthetic zinc ferrite. Overall recoveries of zinc metal from these smelting tests are improved by this refining step; however, the high ratio or iron-aluminum compound to zinc precludes application of the submersible centrifuge on a continuous basis.
Summary and Discussion of Special investigation
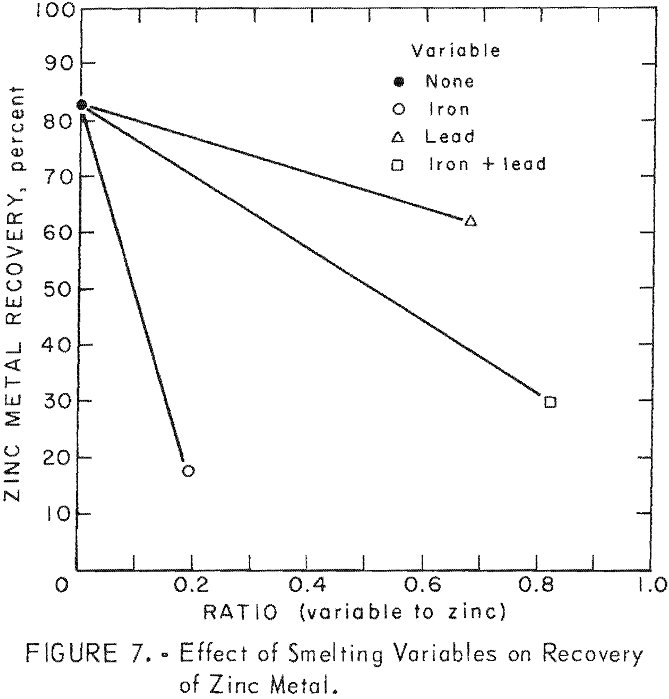
Comparisons of smelting parameters are shown in table 18 and illustrated in figure 7. Smelting charges having iron to zinc ratio of 0.192 reduced recovery of zinc metal from 82.94 to 17.35 percent. Formation of an iron-zinc product was caused by interaction of iron with zinc vapor in the zinc-vapor gas stream and with liquid zinc in the condenser splash medium. Formation and accumulation of this high-melting intermetallic product in the condenser creates an undesirable operating condition which prevents continuous operation. Continuous removal of this product by circulating the splash medium to a settling vessel or container would be difficult and would not improve zinc recovery without refining. An investigation conducted at the College Park Metallurgy Research Center to refine this product on a continuous basis by precipitating iron with aluminum and applying filtration or centrifugation proved unsatisfactory.
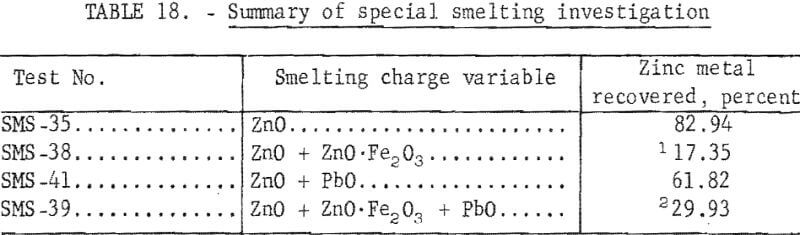
Smelting of a charge having a lead to zinc ratio of 0.68 resulted in a decrease in recovery of zinc metal from 82.94 to 61.82 percent.
The combined effects of zinc ferrite and lead oxide, in a charge having an iron plus lead to zinc ratio of 0.82, were manifested by a decrease in recovery of zinc metal from 82.94 to 29.93 percent. The zinc-rich phase, containing 1.96 percent iron, would require refining to obtain a salable product.
Results of these special smelting tests, designed to differentiate the effects of several smelting variables, revealed that the principal cause for unsatisfactory recovery of zinc metal was the interaction of iron with zinc vapor and with liquid zinc in the condenser. These special smelting tests have established the limitations of slag-resistance open-bath electric smelting of complex charges containing lead, zinc, and iron. Attempts to reduce or eliminate iron transfer to the condenser were not successful because of inherent characteristics of electric smelting. Excessive transfer or iron from the electric smelting furnace to the zinc condenser is attributed to localized super heating in and near the electrical resistance path. Features considered essential to successful operation such as a filtration column, operation with a hot-top charge-column, or introduction of air into the furnace above a charge column to supply the necessary thermal requirements, are either impractical or unsafe.
Summary
A sealed, slag-resistance open-bath, electric smelting furnace was designed, fabricated, and modified to provide necessary smelting requirements. In conjunction with this smelting unit, two distinct condensing systems were designed, fabricated, and tested for condensation of lead and zinc vapor.
All smelting tests gave satisfactory reduction of lead and zinc. Condensation of zinc and lead vapor to metal powder in a water-cooled shock-condensing system was satisfactory. Metal-splash condenser tests resulted in partially successful recovery of zinc metal.
Several major problem were identified. These problems were formation of chemical blue powder, transfer of furnace products such as volatile slag and metal constituents to the condenser, and the formation of iron-zinc inter-metallic compounds. The first problem was partially resolved by supplying the necessary thermal requirements to the gas and zinc vapor stream. Special tests were designed and conducted to differentiate the effects of several smelting variables on the yield of zinc metal. Smelting parameters were conventional electrothermic smelting of zinc oxide, effect of zinc ferrite, effect of lead oxide, and combined effects of zinc ferrite and lead oxide.
Results of these special tests established that the principal cause for unsatisfactory recovery of zinc metal was transfer of iron from the smelting charge to the condenser. Interaction of iron with zinc vapor and with liquid zinc produced iron-zinc intermetallic compounds. X-ray and microprobe analyses identified the constituents and their complex association.
Results of a refining investigation conducted at College Park Metallurgy Research Center indicated that refining by this technique could improve overall recovery of zinc metal. The high ratio of the iron-aluminum compound to zinc prevents application of the submersible centrifuge on a continuous basis. In view of metallurgical and economic considerations, this refining step is unwarranted.
Complete volatilization and transfer of the lead, as a result of localized superheating characteristic of electric smelting, would result in loss of any precious metals. Insufficient lead fall to collect precious metals on the furnace hearth would result in distribution of these metals in condenser metal and discard slag.
The requirement that the mixture of zinc vapor and reduction gas be maintained above its equilibrium temperature precludes operation with a dry-charge column in electric-arc or slag-resistance smelting. A charge could not remain dry when heat transfer through that charge must be largely by radiation and conduction.
